
Concept explainers
A Boron and hydrogen form an extensive family of compounds, and the diagram below shows how they are related by reaction.
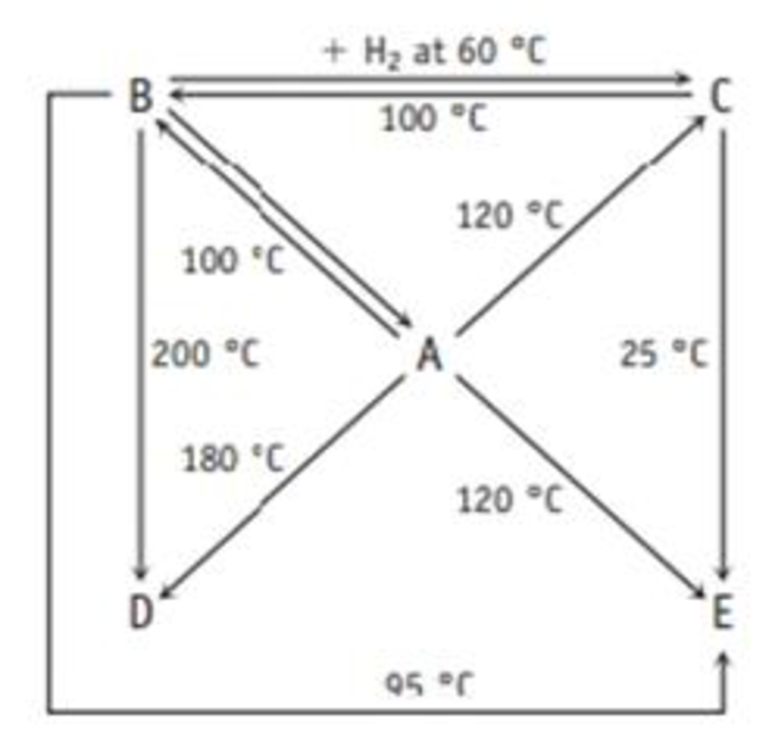
The following table gives the weight percent of boron in each of the compounds. Derive the empirical and molecular formulas of compounds A-E.
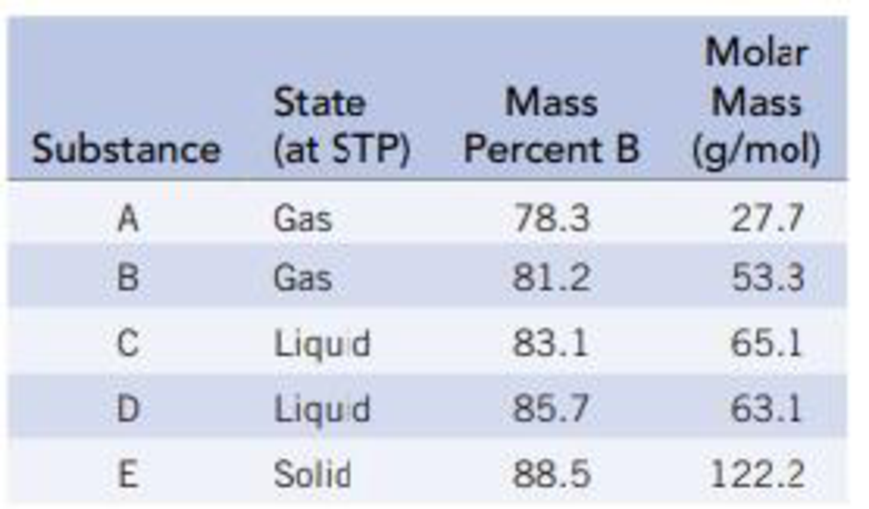
Interpretation: To determine the empirical and molecular formula of given compounds A-E.
Concept introduction:
The empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound.
A molecular formula shows the total number of atoms in a molecule but not their structural arrangement.
Answer to Problem 107GQ
The empirical formula of compound A is
The empirical formula of compound B is
The empirical formula of compound C is
The empirical formula of compound D is
The empirical formula of compound E is
Explanation of Solution
Boron and hydrogen form an extensive family of compounds. Substance A-E contains boron and hydrogen atoms.
The empirical and molecular formula of given compounds A-E is calculated below.
Given:
Substance A is a gaseous compound contains
The empirical formula of substance A is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound A is
The empirical formula molar mass of compound A is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance A by the number calculated above.
Thus, the molecular formula of substance A is
Substance B is a gaseous compound contains
The empirical formula of substance B is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound B is
The empirical formula molar mass of compound B is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance B by the number calculated above.
Thus, the molecular formula of substance B is
Substance C is a liquid compound contains
The empirical formula of substance C is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound C is
The empirical formula molar mass of compound A is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance C by the number calculated above.
Thus, the molecular formula of substance C is
Substance D is a liquid compound contains
The empirical formula of substance D is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound D is
The empirical formula molar mass of compound A is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance D by the number calculated above.
Thus, the molecular formula of substance D is
Substance E is a solid compound contains
The empirical formula of substance E is calculated as,
Convert the mass of boron and hydrogen into moles using molar mass of boron and hydrogen respectively.
Divide each mole value by the smallest number of moles calculated. Round off to the nearest whole number.
Thus, the empirical formula of compound E is
The empirical formula molar mass of compound E is
Divide the molecular formula mass by the empirical formula mass,
Multiply each of the subscripts within the empirical formula of substance E by the number calculated above.
Thus, the molecular formula of substance E is
The empirical formula of compound A is
The empirical formula of compound B is
The empirical formula of compound C is
The empirical formula of compound D is
The empirical formula of compound E is
Want to see more full solutions like this?
Chapter 21 Solutions
Chemistry & Chemical Reactivity
- A sample of a hydrocarbon (a compound consisting of only carbon and hydrogen) contains 2.59 1023 atoms of hydrogen and is 17.3% hydrogen by mass. If the molar mass of the hydrocarbon is between 55 and 65 g/mol, what amount (moles) of compound is present, and what is the mass of the sample?arrow_forwardCalculate the mass in grains of hydrogen present in 2.500 g of each of the following compounds. l type="a"> benzene, C6H6 i>calcium hydride, CaH2 i>ethyl alcohol, C2H5OH i>serine, C3H7O3Narrow_forwardA compound containing xenon and fluorine was prepared by shining sunlight on a mixture of Xe (0.526 g) and excess F2 gas. If you isolate 0.678 g of the new compound, what is its empirical formula?arrow_forward
- Nitrogen fixation in the root nodules of peas and other legumes occurs with a reaction involving a molybdenum-containing enzyme named nitrogenase. This enzyme contains two Mo atoms per molecule and is 0.0872% Mo by mass. Calculate the molar mass of the enzyme.arrow_forwardA 0.025-g sample of a compound composed of boron and hydrogen, with a molecular mass of ~28 amu, burns spontaneously when exposed to air, producing 0.063 g of B2O3. What are the empirical and molecular formulas of the compound?arrow_forward3.98 The characteristic odor of decaying flesh is due to the presence of various nitrogen-containing compounds. One such compound, called putrescine, was analyzed and found to contain 54.49% carbon, 13.72% hydrogen, and 31.78% nitrogen by mass. If the molar mass of putrescine is known to be between 85 and 105, what is its molecular formula?arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





