
Concept explainers
(a)
Interpretation: The following incorrectly named compounds are to be drawn and named correctly.
Concept introduction: Organic compounds contain carbon and hydrogen atoms with their respective
To determine: The correct name of the given compound and its structure is to be drawn.
(a)
Answer to Problem 111AE
Answer
The correct name of the compound is
Explanation of Solution
Explanation
The correct name of the compound is
The structure of
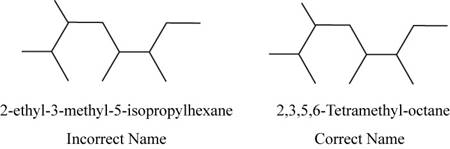
Figure 1
The root name hexane signifies the presence of six carbon atoms.
(b)
Interpretation: The following incorrectly named compounds are to be drawn and named correctly.
Concept introduction: Organic compounds contain carbon and hydrogen atoms with their respective functional groups. When they are named the root term, suffix and prefix are to be remembered. They are named according to the International Union of Pure and Applied Chemistry
To determine: The correct name of the given compound and its structure is to be drawn.
(b)
Answer to Problem 111AE
Answer
The correct name of the compound is
Explanation of Solution
Explanation
The correct name of the compound is
The structure of
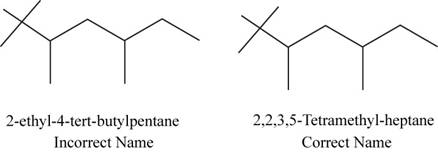
Figure 2
The root name is pentane which signifies the presence of five carbon atoms in a chain,
(c)
Interpretation: The following incorrectly named compounds are to be drawn and named correctly.
Concept introduction: Organic compounds contain carbon and hydrogen atoms with their respective functional groups. When they are named the root term, suffix and prefix are to be remembered. They are named according to the International Union of Pure and Applied Chemistry
To determine: The correct name of the given compound and its structure is to be drawn.
(c)
Answer to Problem 111AE
Answer
The correct name of the compound is
Explanation of Solution
Explanation
The correct name of the compound is
The structure of 3-methyl 4-isopropylpentane with its correct name is,
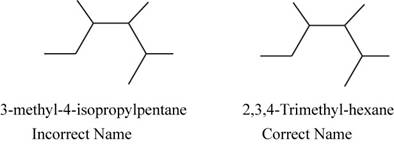
Figure 3
The root name pentane signifies the presence of five carbon atoms in a chain and
(d)
Interpretation: The following incorrectly named compounds are to be drawn and named correctly.
Concept introduction: Organic compounds contain carbon and hydrogen atoms with their respective functional groups. When they are named the root term, suffix and prefix are to be remembered. They are named according to the International Union of Pure and Applied Chemistry
To determine: The correct name of the given compound and its structure is to be drawn.
(d)
Answer to Problem 111AE
Answer
The correct name of the compound is
Explanation of Solution
Explanation
The correct name of the compound is
The structure of
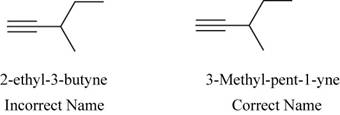
Figure 4
The root name butyne signifies the presence of four carbon atoms with a triple bond in a chain and
Want to see more full solutions like this?
Chapter 21 Solutions
Chemistry: An Atoms First Approach
- Draw the structural formula for each of the following. a. 3-isobutylhexane b. 2,2,4-trimethylpentane, also called isooctane. This substance is the reference (100 level) for octane ratings. c. 2-tert-butylpentane d. The names given in parts a and c are incorrect. Give the correct names for these hydrocarbons.arrow_forwardThe compound CH2=CHCH2CH2CH3 is an example of: a. a pentane. b. a hexene. c. an alkene. d. organic macromolecule.arrow_forwardDraw the structural formula for each of the following. a.3-isobutylhexane b.2,2,4-trimethylpentane, also called isoocta11e. This substance is the reference (100 level) for octane ratings. c. 2-tert-butylpentane d.The names given in parts a and c are incorrect.Give the correct names for these hydrocarbonsarrow_forward
- How would you synthesize each of the following?a. 1,2-dibromopropane from propeneb. acetone (2-propanone) from an alcoholc. tert-butyl alcohol (2-methyl-2-propanol) from an alkene (See Exercise 62.)d. propanoic acid from an alcoholarrow_forwardDraw the structural diagram for the following molecules: a. 5-iodo-deca-2-ene b. 4-ethyl-3-methylhexa-2-enearrow_forwardConsider the reaction in the attached picture: 1. The reaction is an example of ______. a. Addition reaction b. substitution reaction c. elimination reaction 2. The reaction shows _________ a. Oxidation of alcohol to produce an alkene b. Dehydration of alcohol to produce an alkyne c. Dehydration of alcohol to produce an alkenearrow_forward
- Draw the structural diagram for the following molecules: a. 3-methylhepta-1,3,6-triene b. 3-bromo-2-chloro-4-methylhexene c. 5-ethyl-3-methylheptynearrow_forwardHow do structures of these IUPAC names look like? e) 2-Propoxy-1-(2-propoxyethoxy)propanearrow_forwardExplain why each name is incorrect, and then write a correct name. (a) 2-Ethyl-1-propene (b) 4-Methyl-4-hexanearrow_forward
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





