
Concept explainers
(a)
Interpretation:
Why benzene fails to react under the given reaction conditions has to be explained.
Concept Introduction:
Radical reaction: It is reacting to electron octet of valence shell.
A radical can break a bond in another molecular and abstract a partner with an electron, giving substitution in the original molecule.
A radical can added to an
Radical chain reaction:
Initiation reaction: 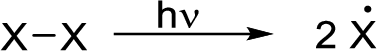
Chain propagation: 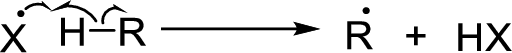
Chain termination:
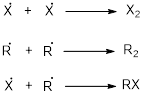
It is a change in enthalpy of a hemolysis reaction at absolute zero where a molecule is broken down into two free radicals.
(b)
Interpretation:
Why the bond dissociation enthalpy of a
Concept Introduction:
Hybridization: It is the mixing atomic orbitals into new hybrid orbitals with different energies, shapes, etc., than the component atomic orbitals suitable for the paring of electrons to from chemical bonds in valance bond theory.
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Organic Chemistry
- Compound A has molecular formula C4H10, and gives two monochlorides, B and C, on photochemical chlorination. Treatment of either of these monochlorides with potassium tert-butoxide gives the same alkene (C4H8) as the product, but B leads to just one isomer of the alkene, D, where C gives D and another isomer of the alkene, E. Treatment of monochlorides B and C with aqueous ethanol gives products F and G, respectively, both of which are of molecular formula C4H10O. What are the names of compounds A-G?arrow_forwardYou are required to synthesize 2-bromopentane from the reaction between an alkene with HBr. Which alkene, 1-pentene or 2-pentene, should you react with HBr in order to get 2-bromopentane? Give an explanation.arrow_forwardGive the skeletal structure of the reacting alkene and the reagents the must eb used to produce BrCH2CHOHCH2Clarrow_forward
- Alkyl halides undergo elimination reactions to produce alkenes by the reacting with strong bases as shown in the following reaction (see image). In general, compounds where the halogen is axial (axial position) are much more reactive than those in which they are in the equatorial position. Taking the above into account: a. Which of the following compounds would give a faster elimination reaction: cis-1-bromo-2-tert-butylcyclohexane or trans-1-bromo-2-tert-butylcyclohexane? Draw the corresponding structures and clearly explain the choice.arrow_forwardWrite chemical structures for compounds A through D in the following sequence of reac- tions. Compounds A and C are alcohols, one of which is cyclicarrow_forwardExplain the Mechanism - Addition of Dichlorocarbene to an Alkene ?arrow_forward
- On being heated with a solution of sodium ethoxide in ethanol, compound A (C7H15Br) yielded a mixture of two alkenes B and C, each having the molecular formula C7H14. Catalytic hydrogenation of the major isomer B or the minor isomer C gave only 3-ethylpentane. Suggest structures for compounds A, B, and C consistent with these observations.arrow_forwardCompound A (C6H12O2) reacts with water, acid, and heatto yield compound B (C5H10O2) and compound C (CH4O).Compound B is acidic. Deduce possible structures of compounds A, B, and Carrow_forwardCompound P (C2H4) which is an alkene undergoes reaction with HCl to produce compound Q (C2H5Cl). Reaction of compound Q with benzene in the presence of AlCl3 as catalyst produces compound R. Then, nitration of compound R in the presence ofnH2SO4 produces two compounds, S and T. But when compound R is reacted with a hot acidified solution of alkaline KMnO4 gives compound U. Deduce the structure of compounds P to U.arrow_forward
- An unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst to give hydrocarbon B. Hydrocarbon A also reacts with OsO4 to give the glycol C. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2COOH, and the other fragment is ketone D (R2C=O). What are the structures of A, B, C and D? Write all reactions.arrow_forwardWhich of the statement is INCORRECT? a. The increase in stability of 2,4-hexadiene over 1,3-hexadiene is due to the increased double bond substitution of the former. b. The stabilization of dienes by conjugation is less pronounced than the aromatic stabilization of benzene. c. Resonance description in alkenes usually involves charge separation. d. Higher energy pi-orbitals often have decreasing number of nodes.arrow_forwardThe rate law for addition of Br2 to an alkene is first orderin Br2 and first order in the alkene. Does this informationsuggest that the mechanism of addition of Br2 to analkene proceeds in the same manner as for addition of HBr?Explain.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
