
(a)
Interpretation:
Electronic configuration of the following metals has to be written –
- (a) Ni (b) Cd (c) Zr (d) Os
Concept Introduction:
Electronic configuration of an atom represents the arrangement of electrons in various energy levels. The electrons are arranged in increasing order of energy levels according to Aufbau principle. It is pictorially represented as –
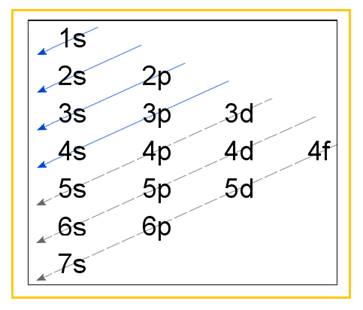
Figure 1
The terms
(b)
Interpretation:
Electronic configuration of the following metals has to be written –
- (a) Ni (b) Cd (c) Zr (d) Os
Concept Introduction:
Electronic configuration of an atom represents the arrangement of electrons in various energy levels. The electrons are arranged in increasing order of energy levels according to Aufbau principle. It is pictorially represented as –
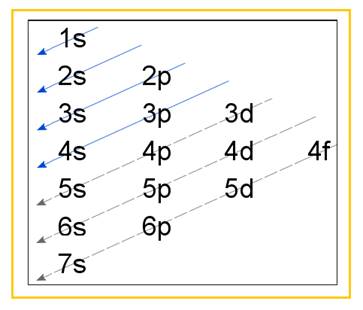
Figure 1
The terms
(c)
Interpretation:
Electronic configuration of the following metals has to be written –
- (a) Ni (b) Cd (c) Zr (d) Os
Concept Introduction:
Electronic configuration of an atom represents the arrangement of electrons in various energy levels. The electrons are arranged in increasing order of energy levels according to Aufbau principle. It is pictorially represented as –
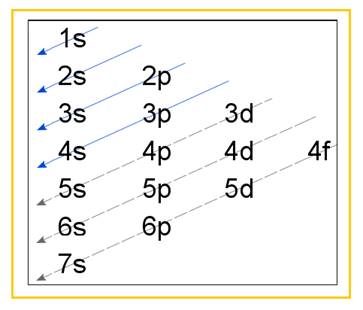
Figure 1
The terms
(d)
Interpretation:
Electronic configuration of the following metals has to be written –
- (a) Ni (b) Cd (c) Zr (d) Os
Concept Introduction:
Electronic configuration of an atom represents the arrangement of electrons in various energy levels. The electrons are arranged in increasing order of energy levels according to Aufbau principle. It is pictorially represented as –
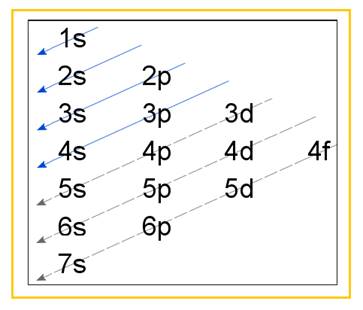
Figure 1
The terms
Want to see the full answer?
Check out a sample textbook solution
Chapter 21 Solutions
Chemistry
- The standard reduction potential for the reaction [Co( H 2 O)6]3+(aq)+e[CO( H 2 O)6]2+(aq) is about 1.8 V. The reduction potential for the reaction [Co( NH 3 )6]3+(aq)+e[Co( NH 3 )6]2+(aq) is +0.1 V. Calculate the cell potentials to show whether the complex ions,. [Co( H 2 O)6]2+ and or [Co( NH 3 )6]2+, can be oxidized to the corresponding Cobalt (III) complex by oxygen.arrow_forwardIn tables of standard reduction potentials that start from large positive values at the top and proceed through 0.0 V to negative values at the bottom, the alkali metals are normally at the bottom of the table. Use your chemical understanding of alkali metals and how they behave in bonding to explain why this is so.arrow_forwardGive the oxidation state of the metal, number of d electrons, and the number of unpaired electrons predicted for [CO(NH3)6]Cl3.arrow_forward
- The halogen oxides and oxoanions are good oxidizing agents. For example, the reduction of bromate ion has an E value of 1.44 V in acid solution: 2BrO3(aq)+12H+(aq)+10eBr2(aq)+6H2O(l) Is it possible to oxidize aqueous 1.0 M Mn2+ to aqueous MnO4 with 1.0 M bromate ion?arrow_forwardThe complex ion NiCL42 has two unpaired electrons, whereas Ni(CN)42 is diamagnetic. Propose structures for these two complex ions.arrow_forwardA constant current of 1.40 amp is passed through an electrolytic cell containing a 0.100 M solution of AgNO3 and a silver anode and a platinum cathode until 2.48 g of silver is deposited. a How long does the current flow to obtain this deposit? b What mass of chromium would be deposited in a similar cell containing 0.100 M Cr3+ if the same amount of current were used?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





