
The compression ratio of an Otto cycle as shown in Figure 21.12 is VA/VB = 8.00. At the beginning A of the compression process, 500 cm3 of gas is at 100 kPa and 20.0°C. At the beginning of the adiabatic expansion, the temperature is TC = 750°C. Model the working fluid as an ideal gas with γ = 1.40. (a) Fill in this table to follow the states of the gas:
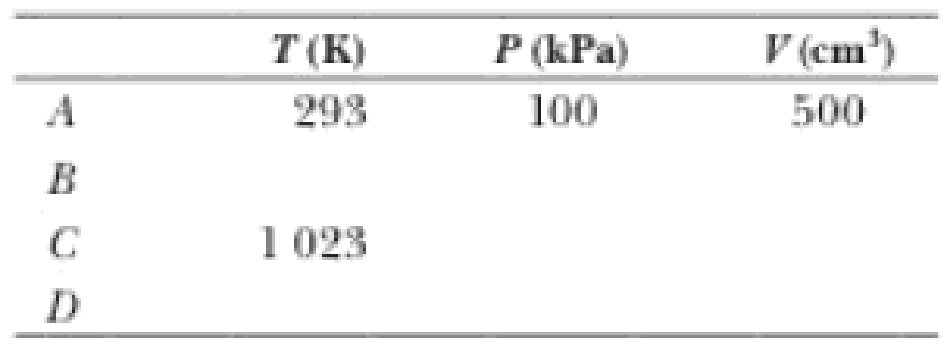
(b) Fill in this table to follow the processes:
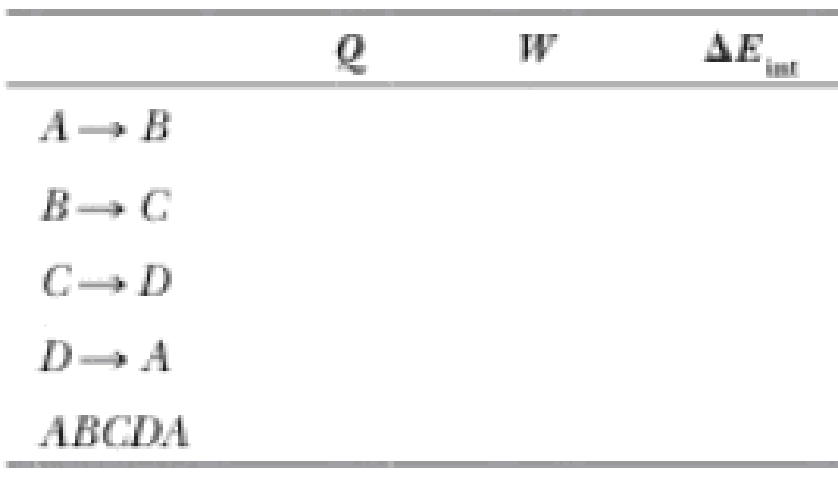
(c) Identify the energy input |Qh|, (d) the energy exhaust |Qc|, and (e) the net output work Weng. (f) Calculate the efficiency. (g) Find the number of crankshaft revolutions per minute required for a one-cylinder engine to have an output power of 1.00 kW = 1.34 hp. Note: The
(a)
The blanks of the table to follow the states of the gas.
Answer to Problem 47CP
The complete table is shown below.
| State |
|
|
|
| A | 293 | 100 | 500 |
| B | 673 |
|
62.5 |
| C | 1023 |
|
62.5 |
| D | 445 | 152 | 500 |
Explanation of Solution
Given info: The compression ratio of an Otto cycle is
Write the expression to calculate the quantity of the gas.
Here,
Substitute
In process
Write the expression to calculate the pressure at point B.
Substitute 8 for
The compression ratio is,
Substitute
Write the expression to calculate the temperature at point B.
Substitute
At state C:
Write the expression to calculate the pressure at point C.
Substitute
State D:
Therefore, the compression ratio is,
Write the expression to calculate the pressure at point D.
Substitute
Write the expression to calculate the temperature at point D.
Substitute
From the above explanation, the complete table is given below.
| State |
|
|
|
| A | 293 | 100 | 500 |
| B | 673 |
|
62.5 |
| C | 1023 |
|
62.5 |
| D | 445 | 152 | 500 |
Conclusion:
Therefore, the complete table is given below.
| State |
|
|
|
| A | 293 | 100 | 500 |
| B | 673 |
|
62.5 |
| C | 1023 |
|
62.5 |
| D | 445 | 152 | 500 |
(b)
The blanks of the table to follow the processes.
Answer to Problem 47CP
The complete table is shown below.
| Process | Q |
|
|
|
|
0 | -162 | 162 |
|
|
149 | 0 | 149 |
|
|
0 | 246 | -246 |
|
|
-65 | 0 | -65 |
|
|
84.3 | 84.3 | 0 |
Explanation of Solution
Given info: The compression ratio of an Otto cycle is
Write the expression to calculate the energy in A to B process.
Substitute
Write the expression of first law of thermodynamics.
Substitute
Write the expression to calculate the energy in B to C process.
Substitute
Write the expression of first law of thermodynamics.
Substitute
Write the expression to calculate the energy in C to D process.
Substitute
Write the expression of first law of thermodynamics.
Substitute
The net work done is,
The net heat energy is,
Substitute
From the above explanation, the complete table is given below.
| Process | Q |
|
|
|
|
0 | -162 | 162 |
|
|
149 | 0 | 149 |
|
|
0 | 246 | -246 |
|
|
-65 | 0 | -65 |
|
|
84.3 | 84.3 | 0 |
Conclusion:
Therefore, the complete table is,
| Process | Q |
|
|
|
|
0 | -162 | 162 |
|
|
149 | 0 | 149 |
|
|
0 | 246 | -246 |
|
|
-65 | 0 | -65 |
|
|
84.3 | 84.3 | 0 |
(c)
The heat input during
Answer to Problem 47CP
The heat input during
Explanation of Solution
Given info: The compression ratio of an Otto cycle is
From part (b), the heat input during
Thus, the heat input during
Conclusion:
Therefore, the heat input during
(d)
The heat exhaust during
Answer to Problem 47CP
The heat exhaust during
Explanation of Solution
Given info: The compression ratio of an Otto cycle is
From part (b)
The heat exhaust during
Thus, the heat exhaust during
Conclusion:
Therefore, the heat exhaust during
(e)
The net work output.
Answer to Problem 47CP
The net work output is
Explanation of Solution
Given info: The compression ratio of an Otto cycle is
From part (b)
The net work output is
Thus, the net work output is
Conclusion:
Therefore, the net work output is
(f)
The thermal efficiency.
Answer to Problem 47CP
The thermal efficiency is
Explanation of Solution
Given info: The compression ratio of an Otto cycle is
Write the expression to calculate the thermal efficiency.
Substitute
Thus, the thermal efficiency is
Conclusion:
Therefore, the thermal efficiency is
(g)
The number of crankshaft revolution per minute.
Answer to Problem 47CP
The number of crankshaft revolution per minute is
Explanation of Solution
Given info: The compression ratio of an Otto cycle is
Write the expression to calculate the output power.
Here,
Substitute
Thus, the number of crankshaft revolution per minute is
Want to see more full solutions like this?
Chapter 21 Solutions
Physics for Scientists and Engineers with Modern Physics
Additional Science Textbook Solutions
Physical Universe
MODERN PHYSICS (LOOSELEAF)
College Physics
Applied Physics (11th Edition)
EBK FUNDAMENTALS OF THERMODYNAMICS, ENH
- The compression ratio of an Otto cycle as shown in Figure 21.12 is VA/VB = 8.00. At the beginning A of the compression process, 500 cm3 of gas is at 100 kPa and 20.0C. At the beginning of the adiabatic expansion, the temperature is TC = 750C. Model the working fluid as an ideal gas with = 1.40. (a) Fill in this table to follow the states of the gas: (b) Fill in this table to follow the processes: (c) Identify the energy input |Qh|, (d) the energy exhaust |Qc|, and (e) the net output work Weng. (f) Calculate the efficiency. (g) Find the number of crankshaft revolutions per minute required for a one-cylinder engine to have an output power of 1.00 kW = 1.34 hp. Note: The thermodynamic cycle involves four piston strokes.arrow_forwardAn idealized diesel engine operates in a cycle known as the air-standard diesel cycle shown in Figure P18.48. Fuel is sprayed into the cylinder at the point of maximum compression, B. Combustion occurs during the expansion B C, which is modeled as an isobaric process. Show that the efficiency of an engine operating in this idealized diesel cycle is e=11(TDTATCTB) Figure P18.48.arrow_forwardA 1.00-mol sample of an ideal monatomic gas is taken through the cycle shown in Figure P18.63. The process AB is a reversible isothermal expansion. Calculate (a) the net work done by the gas, (b) the energy added to the gas by heat, (c) the energy exhausted from the gas by heat, and (d) the efficiency of the cycle. (e) Explain how the efficiency compares with that of a Carnot engine operating between the same temperature extremes. Figure P18.63arrow_forward
- A Carnot engine employs 1.5 mol of nitrogen gas as a working substance, which is considered as an ideal diatomic gas with =7.5 at the working temperatures of the engine. The Carnot cycle goes in the cycle ABCDA with AB being an isothermal expansion. The volume at points A and C of the cycle are 5.0103 m3 and 0.15 L, respectively. The engine operates between two thermal baths of temperature 500 K 300 K. (a) Find the values of volume at B and D. (b) How much heat is absorbed by the gas in the AB isothermal expansion? (c) How much work is done by the gas in the AB isothermal expansion? (d) How much heat is given up by the gas in the CD isothermal expansion? (e) How much work is done by the gas in the CD isothermal compression? (f) How much work is done by the gas in the BC adiabatic expansion? (g) How much work is done by the gas in the DA adiabatic compression? (h) Find the value of efficiency of the engine based on the net and heat input. Compare this value to the efficiency of a Carnot engine based on the temperatures of the baths.arrow_forwardA certain heat engine performs a cycle described by the curve A→B→C→A in the p-V diagram shown in the figure. The segments between the designated points are all straight. The values of the pressures and volumes at the designated points are: VA = 0.91×10-3 m3VB = 3.8×10-3 m3pA = 2.95×106 PapB = 2.34×106 PapC = 1.16×106 Pa What is the net work output of the heat engine, in joules, over a single cycle of operation?arrow_forwardThe temperature of a spark ignition engine operating according to the ideal Otto cycle is T1 = 325 K and pressure P1 = 98 kPa at the beginning of compression. The compression ratio is r = 6, H / Y = 15. The course volume of the cylinder is Vh = 624 cm3, and the number of revolutions is n = 3160 rpm. The lower calorific value of the fuel is 38.2 MJ / kg. Combustion efficiency is y = 0.76 and the number of cylinders is 6. C v = 716.5 J / kg K, R = 287.13 J / kg K. a) Find the thermodynamic coordinates of the cycle. b) Find the thermal efficiency and the indication power of the engine. c) Find the mass of gasoline the engine will use per hour.arrow_forward
- (a) New-age power plants are often times forced to limit hot reservoir temperatures in steam engines to ~280 deg C due to the lack of sufficient heat-resistant materials. Assuming a reversible Carnot steam engine operating at this temperature uses room temperature (20 deg C) as the cold reservoir temperature, determine the total work produced by the system per complete cycle. The volumes for the system at each of the four Carnot states are V1 = 4.0 L, V2 = 16.0 L, V3 = 24.5 L, and V4 = 5.5 L. 1 mol of gas is used with a temperature-independent specific constant-volume heat capacity, CV = 1.9 kJ kg K . Assume the gas is carbon dioxide, with a molar mass of 44.01 g/mol. (b) What is the efficiency, η, of this Carnot engine? (c) What are three physical parameters that could be changed to improve the efficiency of the engine? Explain your answers.arrow_forwardThe PV diagram in the figure below shows a set of thermodynamic processes that make up a cycle ABCDA for a monatomic gas, where AB is an isothermal expansion occurring at a temperature of 355 K. There are 1.35 mol of gas undergoing the cycle with PA = 1.01 ✕ 106 Pa, PB = 5.10 ✕ 105 Pa, and PC = 2.02 ✕ 105 Pa. (a) Find the volumes VA and VB. (b) Find the change in thermal energy during the constant-volume process BC.arrow_forwardA four-cylinder spark-ignition engine has a compression ratio of 10.5, and each cylinder has a maximum volume of 0.4 L. At the beginning of the compression process, the air is at 98 kPa and 37C, and the maximum temperature in the cycle is 2100 K. Assuming the engine to operate on the ideal Otto cycle, determine (a) the amount of heat supplied per cylinder, (b) the thermal efficiency, and (c) the number of revolutions per minute required for a net power output of 45 kW. Assume variable specific heats for air.arrow_forward
- A Carnot cycle is a thermodynamic process consisting of four stages: 1) isothermal expansion; 2) adiabatic expansion; 3) isothermal compression; and 4) adiabatic compression back to the original state. Assuming you have 1.00 mol of a monatomic ideal gas at standard state and the volume is doubling during stage 1 (isothermal expansion), calculate the change in internal energy for this part of the Carnot cycle. A)22.4 J B) 2.27 kJ C)-2.27 kJ D)-1.72 kJ E)0 J For the same system as in Question ^, calculate the change in temperature for the second stage (adiabatic expansion), assuming that the volume doubles yet again (that is, it is twice as large as the volume at the end of stage 1 but four times greater than the initial volume at the beginning of stage A) 0K B) 188K C) -188K D) -110K E) 110Karrow_forwardSuppose a monatomic ideal gas is changed from state A to state D by one of the processes shown on the PV diagram.where P1 = 3.10 and P2 = 6.20. Find the total work done on the gas if it follows the constant-volume path AB followed by the constant-pressure path BCD.arrow_forwardBy operating a reversible thermal machine with an ideal gas as the working substance in a Carnot cycle (see figure) and measuring the ratio Q_F /Q_Q , we can calculate: (a) n, the number of mols of the ideal gas.b) the ratio V₂ /V₃c) the ratio P₁ / P₄d) the ratio (P₁ V₁) / (P₃ V₃)(e) the value of Avogadro's number.f) None of the above.arrow_forward
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning





