
Concept explainers
Glycine has pK2 values of 2.34 and 9.60. At what pH does glycine exist in the forms shown?
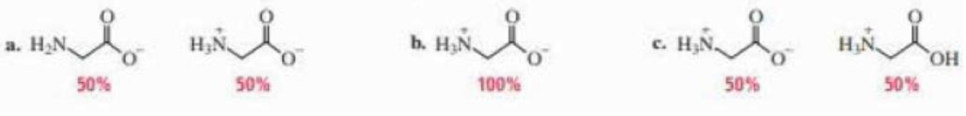
(a)
Interpretation:
The pH at which glycine exists at the given form has to be calculated.
Concept introduction:
The isoelectric point
The value of
Protons are released when the
Answer to Problem 49P
The pH of the glycine for the given form is
Explanation of Solution
In the given form of glycine,
The
The
Therefore,
The pH of the glycine for the given form is
(b)
Interpretation:
The pH at which glycine exists at the given form has to be calculated.
Concept introduction:
The isoelectric point
The value of
Protons are released when the
Explanation of Solution
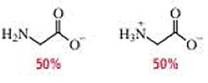
In the given form of glycine,
The amount of positive charge and negative charge are equal or balanced for 100% system.
The isoelectric point (
Therefore,
The pH of the glycine for the given form is
(c)
Interpretation:
The
Concept introduction:
The isoelectric point
The value of
Protons are released when the
Answer to Problem 49P
The pH of the glycine for the given form is
Explanation of Solution
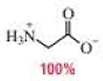
In the glycine form of glycine,
The
The
Therefore,
The pH of the glycine for the given form is
Want to see more full solutions like this?
Chapter 21 Solutions
ORGANIC CHEMISTRY (LL)-W/MOD.MASTERING.
Additional Science Textbook Solutions
Essential Organic Chemistry (3rd Edition)
Chemistry: A Molecular Approach (4th Edition)
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry: Structure and Properties
Chemistry
Chemistry & Chemical Reactivity
- The structure below is a ____________. cerebroside monoglycosyl ceramide glycosphingolipid all are correctarrow_forwardIn 0.1M solution glycine (pKa 9.6) at pH of 9.0, what fraction of glycine has its amino group in the -NH3+ form?arrow_forwardCalculate the degree of unsaturation in each of the following formulas (a) Cholesterol, C27H46o (b) DDT, C14HgC15 (c) Prostaglandin E1, C2oH3405 (d) Caffeine, C8H10N402 (e) Cortisone, C21H28O5 (f) Atropine, C17H23NO3arrow_forward
- Using the template structure of sesamin below, label the sets of equivalent carbons as C1, C2, C3, etcarrow_forwarda. State the glycine species at pKa1 and pKa2. b. Why a pH value is equal to pKa value in the middle of titration? c. State the functional group (s) at pKa1 and pKa2. d. If aspartic acid is used, determine how many pKa values will be obtained and give reasons. e. Compare the pKa of glycine and pKa of acetic acid and state which compound is a strong acid or weak acid. Explain why.arrow_forwardCalculate an approximate pI for Peptide E-G-E-Aarrow_forward
- Pentapeptide has a sequence of APEDS Structure of pentapeptide in its proper ionization state at pH 7.0 what is the pI of pentapeptide?arrow_forwardDraw the predominant ion for Gly-Asp-Glu at its pI.arrow_forwardThe anticoagulant heparin is a polysaccharide that contains alternating residues of -D- glucuronic acid-6- sulfate and N-sulfo-D-glucosamine-6sulfate connected by (1 B 4)- glycosidic linkages. Draw a part of heparin that shows one each of the two residues.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

