
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.14, Problem 8P
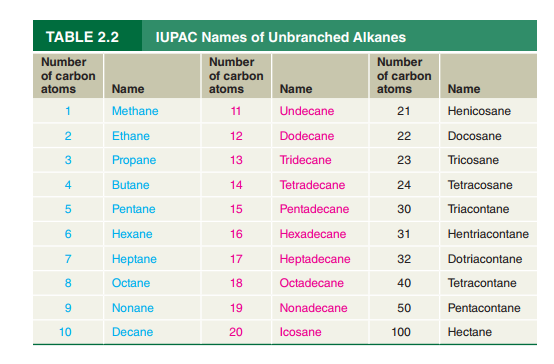
Refer to Table
Beeswax (Figure
formula for hentriacontane.
Octacosane has been found to be present in a certain fossil plant. Write a
condensed structural formula for octacosane.
What is the IUPAC name of the
the cockroach aggregation pheromone?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Draw the expanded structural formula for 1,3-dichlorocyclopentane.An expanded structural formula shows all the atoms of the molecule and all the bonds between the atoms in the molecule.
Please answer ALL parts of the question
A)
Alkanes:
A) Draw the skeletal/line formula for octane, cyclopropane, and pentane.
B) Write the molecular formula for octane, cyclopropane, and pentane.
C) Draw four isomers, in any format, with the formula C6H14.
B)
Draw the following (in any format) and write the molecular formula:
A) 3-iodo-2-methylhexane
B) 1,2-dichloroethane
C) 2-chloro-4-ethylnonane
D) 4-isopropyldecane
E) 2,2,4-tribromo-3-fluorooctane
F) 1,1-difluoro-3-methylcyclohexane
G) 1-ethylcyclobutane
C)
A) Draw the expanded structure of trans-4-chloro-2-pentene
B) 3) Draw 4 skeletal isomers of C7H16 and mane them.
D)
Write the molecular formula of the following:
A) 2-methylbutane
B) 2,3-dibromo-1-pentene
C) 3-bromo-1-pentyne
D) 1,3-diethylbenzene
E) 2,4-diisopropylbenzoic acid
F) 3,3-dichloro-2,2-dimethylhexane
G) 3-fluoro-2-methyl-1-butene
H) 1-ethylcyclopropane
I) meta-propyltoluene
J) Cis-3,4-diiodo-3-heptene
1. Table 1 shows the octane number of some hydrocarbons. Hexane has the lowest octane number of the four compounds listed. Explain why hexane has the lowest octane number.
Table 1
Name
Octane number
Hexane
Cylohexane
Benzene
2,2,4-trimethylpentane
25
83
100
100
2. A molecule of the hydrocarbon C12H26 was cracked to give 2 molecules of ethene, C2H4, one of propene, C3H6 and one other molecule. Write the molecular formula for the one other molecule.
Chapter 2 Solutions
Organic Chemistry - Standalone book
Ch. 2.4 - Prob. 1PCh. 2.7 - Prob. 2PCh. 2.8 - Identify the orbital overlaps of all of the bonds...Ch. 2.9 - The hydrocarbon shown, called vinylacetylene, is...Ch. 2.12 - Prob. 5PCh. 2.12 - Prob. 6PCh. 2.13 - Prob. 7PCh. 2.14 - Refer to Table 2.2 as needed to answer the...Ch. 2.15 - Prob. 9PCh. 2.15 - Prob. 10P
Ch. 2.16 - Prob. 11PCh. 2.17 - Prob. 12PCh. 2.18 - Prob. 13PCh. 2.20 - Prob. 14PCh. 2.21 - Match the boiling points with the appropriate...Ch. 2.22 - Write a balanced chemical equation for the...Ch. 2.22 - Using the data in Table 2.3, estimate the heat of...Ch. 2.22 - Prob. 18PCh. 2.22 - Prob. 19PCh. 2.23 - Prob. 20PCh. 2.23 - Which of the following reactions requires an...Ch. 2 - The general molecular formula for alkanes is...Ch. 2 - Prob. 23PCh. 2 - Prob. 24PCh. 2 - Prob. 25PCh. 2 - What is the hybridization of each carbon in...Ch. 2 - Prob. 27PCh. 2 - Does the overlap of two p orbitals in the fashion...Ch. 2 - Prob. 29PCh. 2 - Aphids secrete an alarm pheromone having the...Ch. 2 - All the parts of this problem refer to the alkane...Ch. 2 - Prob. 32PCh. 2 - Prob. 33PCh. 2 - Prob. 34PCh. 2 - From among the 18 constitutional isomers of C8H18,...Ch. 2 - Give the IUPAC name for each of the following...Ch. 2 - Using the method outlined in Section 2.16, give an...Ch. 2 - Prob. 38PCh. 2 - Write a balanced chemical equation for the...Ch. 2 - The heats of combustion of methane and butane are...Ch. 2 - In each of the following groups of compounds,...Ch. 2 - Given H for the reaction H2(g)+12O2(g)H2O(l)...Ch. 2 - Prob. 43PCh. 2 - Prob. 44PCh. 2 - Prob. 45PCh. 2 - Prob. 46PCh. 2 - Prob. 47PCh. 2 - Compound A undergoes the following reactions:...Ch. 2 - Prob. 49PCh. 2 - Some Biochemical Reactions of Alkanes Alkanes...Ch. 2 - Prob. 51DSPCh. 2 - Some Biochemical Reactions of Alkanes Alkanes...Ch. 2 - Prob. 53DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Constitutional isomers are compounds which have the same molecular formula but different structural formulae. They are different compounds with different physical and chemical properties.a. Rearrange your model of n-hexane to make as many possible isomers of C6H14 as you can. Draw the structural formula and write down the IUPAC name for each isomer that you make.b. Constitutional isomers can also have different functional groups. Make all possible isomers of C3H8O. Write down the structural formulae and IUPAC names for all the compounds you make. Hint: Consider alcohol and ether functional groups.arrow_forwardIllustrate the chemical structural formula for 3-methyl-3-ethylpentane. (b) Identify its chemical family as an isomer. (c) Provide the balanced chemical reaction equation for the combustion of one mole of this fuel with an equivalence ratio of ϕ=0.735arrow_forwardAnswer the following questions. (Be guided with the rubric below) Fossil fuels are the raw materials for many consumer products. Do you think it is wise to continue to use the limited supplies of fossil fuels as an energy source? Explain. Fruits are sometimes picked while green and transported long distances, after which they are exposed to ethene gas, which speeds the ripening process. Research and write a short paper detailing the advantages and disadvantages of using ethene to speed the ripening of fruits.arrow_forward
- You are trying to monochlorinate 2,4,4-trimethylhexane to form a compound with the molecular formula C9H19Cl. Answer following question: A. Draw the structures of all the monochlorinated isomers which have the formula C9H19Cl and are derived from 2,4,4-trimethylhexane. B. Give the IUPAC name for each of the structures you drew in part A. C. For each of the structures you drew in part A circle any asymmetric carbon atoms.arrow_forwardDraw all of the possible structural isomers (structural formula or condensed formula) for butane, pentane, hexane and heptane and name them. Upload pictures of your answers. C4H10 (Hint: there are 2 possible isomers) C5H12 (Hint: there are 3 possible isomers) C6H14 (Hint: there are 5 possible isomers) C7H16 (Hint: there are 9 possible isomers)arrow_forwardOrganic compounds may have characteristic odors as well as other characteristic physical properties. For example, the distinct odor of the seashore at low tide results in part from the presence of dimethyl sulfide (CH3SCH3), a molecule with a similar structure to dimethyl ether (CH3OCH3). Ethanethiol (CH3CH2SH), also called mercaptan, is an isomer of dimethyl sulfide with a much less pleasant odor.The table lists four related compounds and their enthalpies of vaporization (ΔH°vap) in kJ/mol. Compound ΔH°vap (kJ/mol) CH3OCH3 23 CH3SCH3 28 CH3CH2SH 27.5 CH3CH2OH 42 Rank the following compounds in order of increasing strength of their intermolecular forces, given the ΔH°vap listed for each. Place the compound with the strongest intermolecular forces (IMFs) at the top of the list. (Strongest to weaknest). Why is ΔHºvap for CH3SCH3 greater than ΔHºvap for CH3OCH3? A. CH3OCH3 is more polar. B. CH3SCH3 has stronger dipole–dipole attractions. C. CH3OCH3 can form…arrow_forward
- introduced the idea of structural isomerism,with 1-propanol and 2-propanol as examples. Determinewhich of these properties would distinguish these two substances:(a) boiling point, (b) combustion analysis results,(c) molecular weight, (d) density at a given temperature andpressure. You can check on the properties of these two compoundsin Wolfram Alpha (http://www.wolframalpha.com/)or the CRC Handbook of Chemistry and Physics.arrow_forwardSelect the correct IUPAC name for the straight‑chain alkane. CH3(CH2)10CH3 The alkane is named: nonane octane heptane dodecanearrow_forwardBased on your knowledge of boiling point trends in alkanes, predict which compound has the higher boiling point, butane or 2-methylpropane (isobutane). Based on your knowledge of boiling point trends in alkanes, predict which compound has the higher boiling point, butane or 2-methylpropane (isobutane). a. isobutane would have a higher boiling point, since the greater the surface area, the higher the boiling point. b. Butane would have a higher boiling point, since the greater the surface area, the higher the boiling point. c. Butane would have a higher boiling point, since the smaller the surface area, the higher the boiling point. d. Isobutane would have a higher boiling point, since the smaller the surface area, the higher the boiling point.arrow_forward
- 1. Give two organic compounds. The first compound must be a synthetic or man-made organic compound and the second compound must be a natural product or organic compound from natural sources (medicinal plant, coral, animal such as snail or microorganism such as bacteria or fungi). Compounds must have equal to or more than twenty carbon atoms. 2. Your chosen organic compound must have application in the management of human health or veterinary medicine. (for example for the treatment of pain or bacterial infection in human and animal).arrow_forward1. The torch used to start the modern Olympic Games uses a mixture ofpropane and butane. When propane and butane burn in the air, they produce heat, energy, and a flame. A) Propane and butane are chemical compounds. Name the two elements that make these compounds. B) State the name of the compounds that contain the elements in part A C) To which homologous series do propane and butane belong?arrow_forwardDraw an organic structure of an alkane that has: a) five carbons and all secondary b) eight carbons with only primary hydrogens c) seven carbons with 2 isopropyl groupsarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License