
(a)
Interpretation: The structures of the final products formed in the following reactions are to be stated.
Concept introduction: Organic compounds are synthesized through organic reactions. The different types of reactions in
To determine: The structure of
(a)
Explanation of Solution
Explanation
The structure of
The structure of
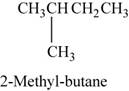
Figure 1
The given reactant is
(b)
Interpretation: The structures of the final products formed in the following reactions are to be stated.
Concept introduction: Organic compounds are synthesized through organic reactions. The different types of reactions in organic chemistry are elimination reaction, substitution reaction, addition reactions and many more. Addition reactions are takes place when two or more reactants combine to form a single product. Elimination reactions occur when a reactant broke down into two or more products and the substitution reactions takes place by an exchange in the reactants.
To determine: The structure of
(b)
Explanation of Solution
Explanation
The structure of
The structure of
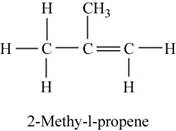
Figure 2
The given reactant reacts with water to form tertiary alcohol as the major product. As tertiary alcohols are formed by the dehydration of
(c)
Interpretation: The structures of the final products formed in the following reactions are to be stated.
Concept introduction: Organic compounds are synthesized through organic reactions. The different types of reactions in organic chemistry are elimination reaction, substitution reaction, addition reactions and many more. Addition reactions are takes place when two or more reactants combine to form a single product. Elimination reactions occur when a reactant broke down into two or more products and the substitution reactions takes place by an exchange in the reactants.
To determine: The two possible structures of
(c)
Explanation of Solution
Explanation
The two possible structures of
The two possible structures of
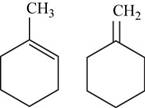
Figure 3
The given chemical formula is
(d)
Interpretation: The structures of the final products formed in the following reactions are to be stated.
Concept introduction: Organic compounds are synthesized through organic reactions. The different types of reactions in organic chemistry are elimination reaction, substitution reaction, addition reactions and many more. Addition reactions are takes place when two or more reactants combine to form a single product. Elimination reactions occur when a reactant broke down into two or more products and the substitution reactions takes place by an exchange in the reactants.
To determine: The structure of hydrocarbon reacted with
(d)
Explanation of Solution
Explanation
The structure of hydrocarbon is shown in Figure 4.
The structure of hydrocarbon is,
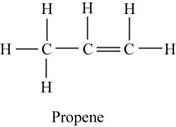
Figure 4
The given hydrocarbon reacts with water which is further oxidized to give acetone. Therefore, the given hydrocarbon should be alkene which is propene as the major product is
(e)
Interpretation: The structures of the final products formed in the following reactions are to be stated.
Concept introduction: Organic compounds are synthesized through organic reactions. The different types of reactions in organic chemistry are elimination reaction, substitution reaction, addition reactions and many more. Addition reactions are takes place when two or more reactants combine to form a single product. Elimination reactions occur when a reactant broke down into two or more products and the substitution reactions takes place by an exchange in the reactants.
To determine: The possible structures for
(e)
Explanation of Solution
Explanation
The first possible structure for
The first possible structure for
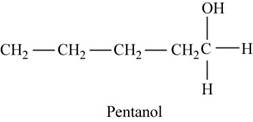
Figure 5
The major product for this reaction is
The second possible structure for
The second possible structure for
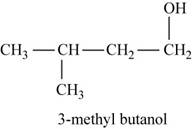
Figure 6
The isomer for the given reactant
The third possible structure for
The third possible structure for
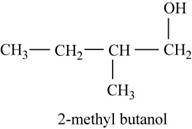
Figure 7
The isomer for the given reactant
The fourth possible structure for
The fourth possible structure for
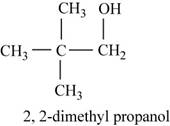
Figure 8
The isomer for the given reactant
Want to see more full solutions like this?
Chapter 22 Solutions
Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





