
Concept explainers
(a)
Interpretation:
The structure of diethyl malonate showing acidic hydrogens is to be stated. The reason as to why it is more acidic than ordinary ester is to be stated.
Concept introduction:
Acidic hydrogen is defined as hydrogen that carries positive charge when the acid dissociates. The acidity in esters depends upon the stability of enolate ion. The higher is the stability of enolate ion higher is the acidity.
Answer to Problem 22.1P
The structure of the diethyl malonate showing acidic hydrogens is given below.
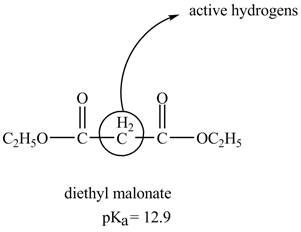
The diethyl malonate is more acidic than ordinary ester because its conjugate base is stabilized by delocalization of negative charge as shown below.
Explanation of Solution
In diethyl malonate the methylene group is surrounded by two carbonyl groups as shown below.
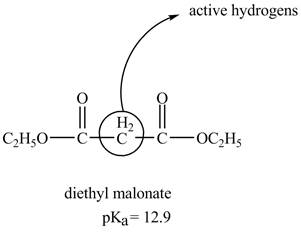
Figure 1
This active hydrogen is abstracted by base and generates a negative charge. The negative charge is delocalized between carbon and oxygen. This delocalization stabilizes the enolate ion. However, the presence of two carbonyl group increases the polar effect and stabilize the enolate ion. The diethyl malonate is more acidic than ordinary ester as its conjugate base is stabilized by delocalization of negative charge.
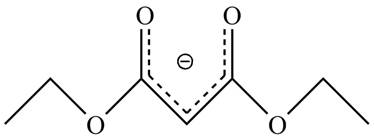
The acidity of the carbonyl compound is directly proportional to the stability of the enolate ion. The conjugate base stabilized by delocalization of diethyl malonate is shown below.
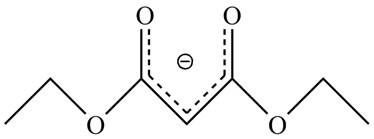
Figure 2
The structure of the diethyl malonate showing acidic hydrogens is given in Figure 2. The diethyl malonate is more acidic than ordinary ester due to delocalization of negative charge in its conjugate base.
(b)
Interpretation:
The structure of ethyl acetoacetate showing acidic hydrogens is to be stated. The reason as to why it is more acidic than ordinary ester is to be stated.
Concept introduction:
Acidic hydrogen is defined as hydrogen carry positive charge when the acid dissociates. The acidity in esters depends upon the stability of enolate ion. The higher is the stability of enolate ion higher is the acidity.
Answer to Problem 22.1P
The structure of ethyl acetoacetate showing acidic hydrogens is given below.
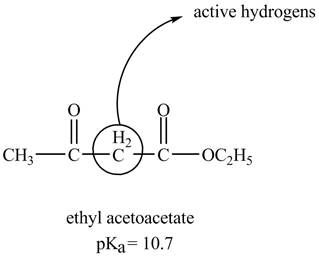
The ethyl acetoacetate is more acidic than ordinary ester because its conjugate base is stabilized by delocalization of negative charge as shown below.
Explanation of Solution
In ethyl acetoacetate, the methylene group is surrounded by two carbonyl groups as shown below.
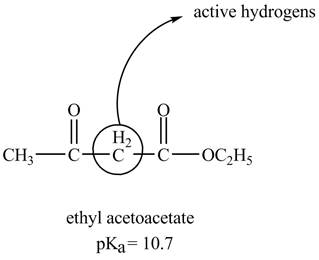
Figure 3
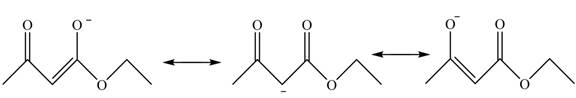
This active hydrogen is abstracted by base and generates a negative charge. The negative charge is delocalized between carbon and oxygen. This delocalization stabilizes the enolate ion. However, the presence of two carbonyl groups increases the polar effect and stabilize the enolate ions. The conjugate base stabilized by delocalization of ethyl acetoacetate is shown below.

Figure 4
The ethyl acetoacetate is more acidic than ordinary ester as its conjugate base is stabilized by delocalization of negative charge.
The structure of ethyl acetoacetate is shown in Figure 3 and the acidic character of ethyl acetoacetate is shown in Figure 4.
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry
- Treatment of pentanedioic (glutaric) anhydride with ammonia at elevated temperature leads to a compound of molecular formula C5H7NO2. What is the structure of this product? [Hint: You need to think about the reactivity not only of acid anhydrides but also of amides and carboxylic acids]arrow_forwardFollowing is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forwardFollowing is a retrosynthetic analysis for an intermediate in the industrial synthesis of vitamin A. (a) Addition of one mole of HCl to isoprene gives 4-chloro-2-methyl-2-butene as the major product. Propose a mechanism for this addition and account for its regioselectivity. (b) Propose a synthesis of the vitamin A precursor from this allylic chloride and ethyl acetoacetate.arrow_forward
- Treatment of salicylaldehyde (2-hydroxybenzaldehyde) with bromine in glacial acetic acid at 0°C gives a compound with the molecular formula C7H4Br2O2, which is used as a topical fungicide and antibacterial agent. Propose a structural formula for this compoundarrow_forwardEach of the following reactions has been carried out under conditions such that disubstitution or trisubstitution occurred. Identify the principal organic product in each case. (a) Nitration of p-chlorobenzoic acid (dinitration) (b) Bromination of aniline (tribromination) (c) Bromination of o-aminoacetophenone (dibromination) (d) Bromination of p-nitrophenol (dibromination) (e) Reaction of biphenyl with tert-butyl chloride and iron(III) chloride (dialkylation) (f) Sulfonation of phenol (disulfonation)arrow_forwardThe compound acetophenone has a very similar molar mass to that of benzoic acid and benzamide. However, acetophenone has a much lower m.p. (20 °C) than both such that, by contrast, it is a liquid at room temperature. By considering intermolecular forces and comparing functional group structure, account for this big difference in physical properties.arrow_forward
- Explain how benzaldehyde and dimedone reacts with each other, and then with the aminotriazole to form compound 1a in the presence of an acid catalyst. Provide a detailed reaction mechanism. During the development of the optimized procedure for the experiment, it was found out that compound 1b can also be produced from the same set of starting materials. Propose a detailed reaction mechanism for the formation of 1b. Explain your answer. What factor/s may drive the formation of 1b over 1a?arrow_forwardWhen a mixture of ketone 11 and \alpha, \beta - unsaturated carbonyl 12 are exposed to substoichiometric amounts of NaOMe, product B is obtained selectively. Subsequent treatment of B with excess NaOMe leads to the exclusive formation of tricyclic product 13. (1) Suggest a structure for B and provide a reason for its selective formation. (ii) Provide a reaction mechanism for the formation of 13, which accounts for its selective formation.arrow_forwardWhen 3-bromopyridine is used in this reaction, stronger reaction conditions arerequired and a mixture of 3-aminopyridine and 4-aminopyridine results. Propose amechanism to explain this curious result.arrow_forward
- When the heterocycles pyridine (1) and pyrrole (2) are treated with the strong base methyllithium, pyrrole reacts to produce a lithium amide while pyridine does not react.arrow_forwardHeating toluene in the presence of KMnO4 followed by acification with HCl, converts toluene into benzoic acid. Using this information and any other reactions discussed in this course, show a sequence of reactions showing howing toluene can be converted to p-aminobenzoic acid.arrow_forwardThe pKa values of the carboxylic acid groups of oxaloacetic acid are 2.22 and 3.98.a. Which carboxyl group is the stronger acid?b. The amount of hydrate present in an aqueous solution of oxaloacetic acid depends on the pH of the solution: 95% at pH 0, 81% at pH 1.3, 35% at pH3.1, 13% at pH 4.7, 6% at pH 6.7, and 6% at pH 12.7. Explain this pH dependence.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

