
Concept explainers
Interpretation:
The validation corresponding to the fact that data of carbon monoxide coverage and pressure follows a Langmuir isotherm is to be stated. The equilibrium constant for the adsorption process is to be stated.
Concept introduction:
The isotherm model that describes the adsorption process, if an adsorbate acts as an ideal gas at the isothermal situation is known as Langmuir adsorption isotherm model. In the given isothermal situation, the partial pressure of the adsorbate is related to the volume of the adsorbate which is adsorbed on the solid adsorbent.
Answer to Problem 22.47E
The occurrence of the linear straight line between
The equilibrium constant for the adsorption process is
Explanation of Solution
The given temperature is
The given data of carbon monoxide coverage and pressure in torr is mentioned in the table below.
| Coverage | Pressure (torr) |
The concentration of carbon monoxide is calculated by the formula given below.
Where,
•
•
•
•
•
•
The value of universal gas constant is
| Pressure | Coverage | |||
The graph between
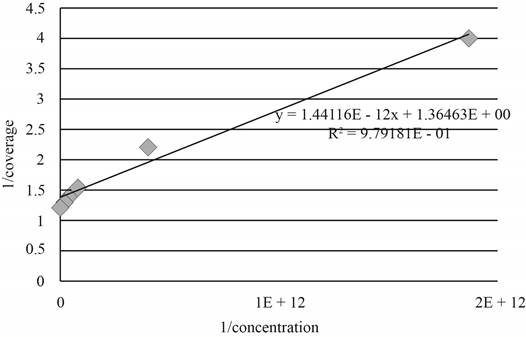
Figure 1
As, the plot between
The value of slope is predicted as
The value of equilibrium constant is calculated by the expression given below.
Substitute the value of slope in the above expression.
Therefore, the value of equilibrium constant is
The given data of carbon monoxide coverage and pressure follows a Langmuir isotherm.
The equilibrium constant for the adsorption process is
Want to see more full solutions like this?
Chapter 22 Solutions
Physical Chemistry
- One of the chemical controversies of the nineteenth century concerned the element beryllium (Be). Berzelius originally claimed that beryllium was a trivalent element (forming Be3+ ions) and that it gave an oxide with the formula Be2O3. This resulted in a calculated atomic mass of 13.5 for beryllium. In formulating his periodic table, Mendeleev proposed that beryllium was divalent (forming Be2+ ions) and that it gave an oxide with the formula Be2O3. This assumption gives an atomic mass of 9.0. In 1894, A. Combes (Comptes Rendus 1894, p. 1221) reacted beryllium with the anion C5H7O2and measured the density of the gaseous product. Combess data for two different experiments are as follows: I II Mass 0.2022 g 0.2224 g Volume 22.6 cm3 26.0 cm3 Temperature 13C 17C Pressure 765.2 mm Hg 764.6 mm If beryllium is a divalent metal, the molecular formula of the product will be Be(C5H7O2)2; if it is trivalent, the formula will be Be(C5H7O2)3. Show how Combess data help to confirm that beryllium is a divalent metal.arrow_forwardAll collisions (in chemistry) involving gases are considered to be elastic collisions. True Falsearrow_forward13 16 19 22 A sample of N₂(g) effuses from a container in 30 s. How long would it take the same amount of NH3(g) to effuse from the same container under identical conditions? 39 s 18 S 23 s 81 sarrow_forward
- Given the BET isotherm plot is y = 123.961 x — 1.181. The surface area of the activated carbon was determined as 85±7 m2 /g. What was the mass of their sample of activated carbon for the adsorption of N2 gas?arrow_forwardA- Iron was inherited at 912 C by the effect of heating from a BCC crystalline structure to a crystalline type (FC C) During this fusion, a change occurred in the atomic radius R = 0.1258nm to R = 0.1289nm. Calculate the ratio of the volume change for this reaction? Is this reaction increasing or decreasing?arrow_forwardWhich of the following molecules only experiences the dispersion (van der Waal's/London) force? hydrobromic acid (gas phase) elemental bromine chlorine-bromide nitrogen tribromidearrow_forward
- At its critical point, ammonia has a density of 0.235 g cm-3 .You have a special thick-walled glass tube that has a10.0-mm outside diameter, a wall thickness of 4.20 mm, anda length of 155 mm. How much ammonia must you sealinto the tube if you wish to observe the disappearance of themeniscus as you heat the tube and its contents to a temperature higher than 132.23°C, the critical temperature?arrow_forwardHow does hydraulic fracturing differ from previously used techniques for the recovery of natural gas from the earth?arrow_forwardHeavy water, D2O (molar mass = 20.03 g mol-1). can be separated from ordinary water, H2O (molar mass = 18.01), as a result of the difference in the relative rates of diffusion of the molecules in the gas phase. Calculate the relative rates of diffusion of H2O and D2O.arrow_forward
- What is the ratio of diffusion rates for nitric oxide(NO) and nitrogen tetroxide (N2O4) ? A. 0.326 B. 0.571 C. 1.751 D. 3.066arrow_forwardAs weather balloons rise from the earths surface, the pressure of the atmosphere becomes less, tending to cause the volume of the balloons to expand. However, the temperatura is much lower in the upper atmosphere than at sea level. Would this temperatura effect tend to make such a balloon expand or contract? Weather balloons do, in fact, expand as they rise. What does this tell you?arrow_forwardSound velocity is usually larger in solids than in liquids and gases because - 1) solids are more elastic than liquids and gases 2) solids are less elastic than liquids and gases 3) volumetric expansion coefficients of liquids and gases are higher than solids 4) frequency of sound waves decreases in solids due to Doppler shiftarrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





