
A 1.00-mol sample of an ideal gas (γ = 1.40) is carried through the Carnot cycle described in Figure 22.11. At point A, the pressure is 25.0 atm and the temperature is 600 K. At point C, the pressure is 1.00 atm and the temperature is 400 K. (a) Determine the pressures and volumes at points A, B, C, and D. (b) Calculate the net work done per cycle.
(a)
The pressure and volume at points
Answer to Problem 22.81CP
For the point
Explanation of Solution
Given data: The number of mole is
Consider the figure of Carnot cycle,
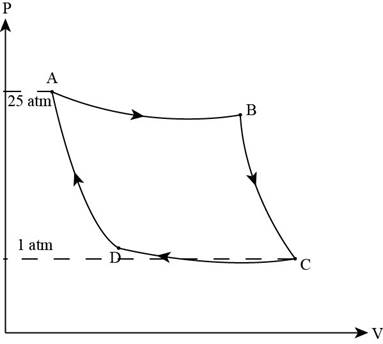
Figure (1)
From the figure, it is clear that the process from
Apply the ideal gas equation for volume at
Here,
The value of ideal gas constant is
Substitute
Thus, the volume at point
Apply the ideal gas equation for volume at
Here,
Substitute
Thus, the volume at point
For the adiabatic process:
The condition is,
Here,
Substitute
Thus, the volume of gas at point
The formula for the pressure at point
Substitute
Thus, the pressure at point
For the adiabatic process:
The condition is,
Here,
Substitute
Thus, the volume of gas at point
The formula for the pressure at point
Substitute
Conclusion:
Therefore, For the point
(b)
The net work done per cycle.
Answer to Problem 22.81CP
The net work done per cycle is
Explanation of Solution
The formula for the net work done per cycle is,
Substitute
Conclusion:
Therefore, the net work done per cycle is
Want to see more full solutions like this?
Chapter 22 Solutions
Physics for Scientists and Engineers, Technology Update (No access codes included)
- At point A in a Carnot cycle, 2.34 mol of a monatomic ideal gas has a pressure of 1 4000 kPa, a volume of 10.0 L, and a temperature of 720 K. The gas expands isothermally to point B and then expands adiabatically to point C, where its volume is 24.0 L. An isothermal compression brings it to point D, where its volume is 15.0 L. An adiabatic process returns the gas to point A. (a) Determine all the unknown pressures, volumes, and temperatures as you f ill in the following table: (b) Find the energy added by heat, the work done by the engine, and the change in internal energy for each of the steps A B, B C, C D, and D A (c) Calculate the efficiency Wnet/|Qk|. (d) Show that the efficiency is equal to 1 - TC/TA, the Carnot efficiency.arrow_forwardAn idealized diesel engine operates in a cycle known as the air-standard diesel cycle shown in Figure P18.48. Fuel is sprayed into the cylinder at the point of maximum compression, B. Combustion occurs during the expansion B C, which is modeled as an isobaric process. Show that the efficiency of an engine operating in this idealized diesel cycle is e=11(TDTATCTB) Figure P18.48.arrow_forwardThe compression ratio of an Otto cycle as shown in Figure 21.12 is VA/VB = 8.00. At the beginning A of the compression process, 500 cm3 of gas is at 100 kPa and 20.0C. At the beginning of the adiabatic expansion, the temperature is TC = 750C. Model the working fluid as an ideal gas with = 1.40. (a) Fill in this table to follow the states of the gas: (b) Fill in this table to follow the processes: (c) Identify the energy input |Qh|, (d) the energy exhaust |Qc|, and (e) the net output work Weng. (f) Calculate the efficiency. (g) Find the number of crankshaft revolutions per minute required for a one-cylinder engine to have an output power of 1.00 kW = 1.34 hp. Note: The thermodynamic cycle involves four piston strokes.arrow_forward
- A 1.00-mol sample of an ideal monatomic gas is taken through the cycle shown in Figure P18.63. The process AB is a reversible isothermal expansion. Calculate (a) the net work done by the gas, (b) the energy added to the gas by heat, (c) the energy exhausted from the gas by heat, and (d) the efficiency of the cycle. (e) Explain how the efficiency compares with that of a Carnot engine operating between the same temperature extremes. Figure P18.63arrow_forwardA certain gasoline engine is modeled as a monatomic ideal gas undergoing an Otto cycle, represented by the p-V diagram shown in the figure. The initial pressure, volume, and temperature are p1 = 1.05 × 105 Pa, V1 = 0.035 m3, and T1 = 290 K, respectively. a)The first step in the Otto cycle is adiabatic compression. Enter an expression for the work performed on the gas during the first step, in terms of V1, V2, and p1. b) Calculate the work performed on the gas during the first step, in joules, for V2 = V1/9.4. c)Calculate the temperature of the gas, in kelvins, at the end of the first step.arrow_forwardA certain gasoline engine is modeled as a monatomic ideal gas undergoing an Otto cycle, represented by the p-V diagram shown in the figure. The initial pressure, volume, and temperature are p1 = 1.05 × 105 Pa, V1 = 0.035 m3, and T1 = 290 K, respectively. a)The first step in the Otto cycle is adiabatic compression. Enter an expression for the work performed on the gas during the first step, in terms of V1, V2, and p1. b) Calculate the temperature of the gas, in kelvins, at the end of the first step. c)The fourth and last step in the Otto cycle is isochoric cooling to the initial conditions. Find the amount of heat, in joules, that is discharged by the gas during the fourth step.arrow_forward
- Consider a monatomic ideal gas operating through the Carnot cycle. The initial volume of the gas is V1 = 130 × 10-3 m3. What types of processes are going on for each step in this process? During the isothermal compression step, the volume of gas is reduced by a factor of 4. In the adiabatic heating step, the temperature of the gas is doubled. What is the volume at point 3, in cubic meters?arrow_forwardA sample containing 1.00 kmol of helium (treated as an ideal gas) is putthrough the cycle of operations shown in the figure. BC is an isotherm,and pA = 1.00 atm, VA = 22.4 m3, pB = 2.00 atm. a) Calculate the temperatures TA, TB and volume VC. b) Calculate the work done during the cycle. Recall the expression for work done during an isothermalprocess.arrow_forwardThe PV diagram in the figure below shows a set of thermodynamic processes that make up a cycle ABCDA for a monatomic gas, where AB is an isothermal expansion occurring at a temperature of 355 K. There are 1.35 mol of gas undergoing the cycle with PA = 1.01 ✕ 106 Pa, PB = 5.10 ✕ 105 Pa, and PC = 2.02 ✕ 105 Pa. (a) Find the volumes VA and VB. (b) Find the change in thermal energy during the constant-volume process BC.arrow_forward
- The figure shows a reversible cycle through which 1.00 mole of a monatomic ideal gas is taken. Process bc is an adiabatic expansion, with pb = 5.80 atm and Vb = 1.00 x 10-3 m3. For the cycle, find (a) the energy added to the gas as heat, (b) the energy leaving the gas as heat, (c) the net work done by the gas, and (d) the efficiency of the cycle.arrow_forwardConsider a process that uses n moles of a monatomic ideal gas operating through a Carnot cycle. The initial temperature and pressure of the gas are T1 and P1, respectively. Consider steps 1 → 2, 2 → 3, 3 → 4, and 4 → 1. a. In the adiabatic heating, the temperature of the gas is doubled. Write an expression for the volume V3 after this step in terms of V1. b. Write an expression for the volume V4 in terms of V1.arrow_forwardThree Carnot engines operate between temperature limits of (a) 400 and 500 K, (b) 500 and 600 K, and (c) 400 and 600 K. Each engine extracts the same amount of energy per cycle from the high-temperature reservoir. Rank the magnitudes of the work done by the engines per cycle, greatest first.arrow_forward
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning




