
Interpretation:
Reason for the preferential of nitration of pyridine at 3rd position has to be given and also resonance contributors for the intermediate formed by attack of
Explanation of Solution
Pyridine is a base, so in the presence of acid it will be protonated. For nitration at the 3rd position, the additional positive charge is delocalized on three carbon atoms of the pyridine ring. In this case, none of the resonance structures has a +2 charge on the same atom.
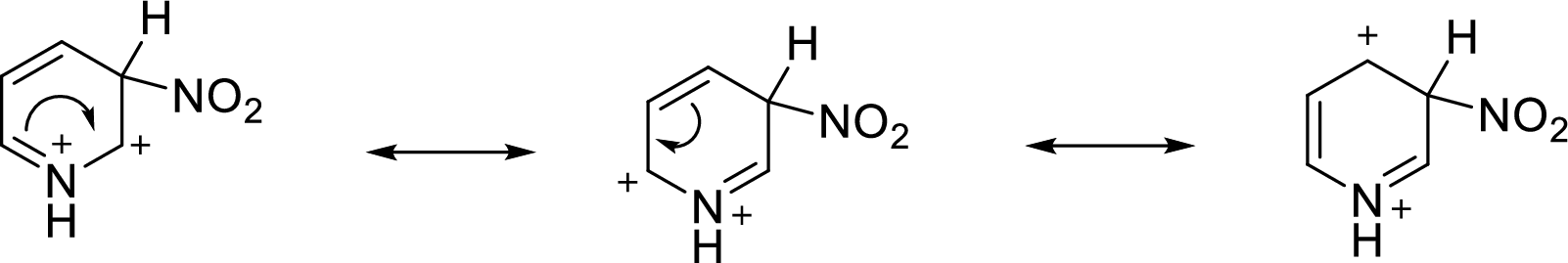
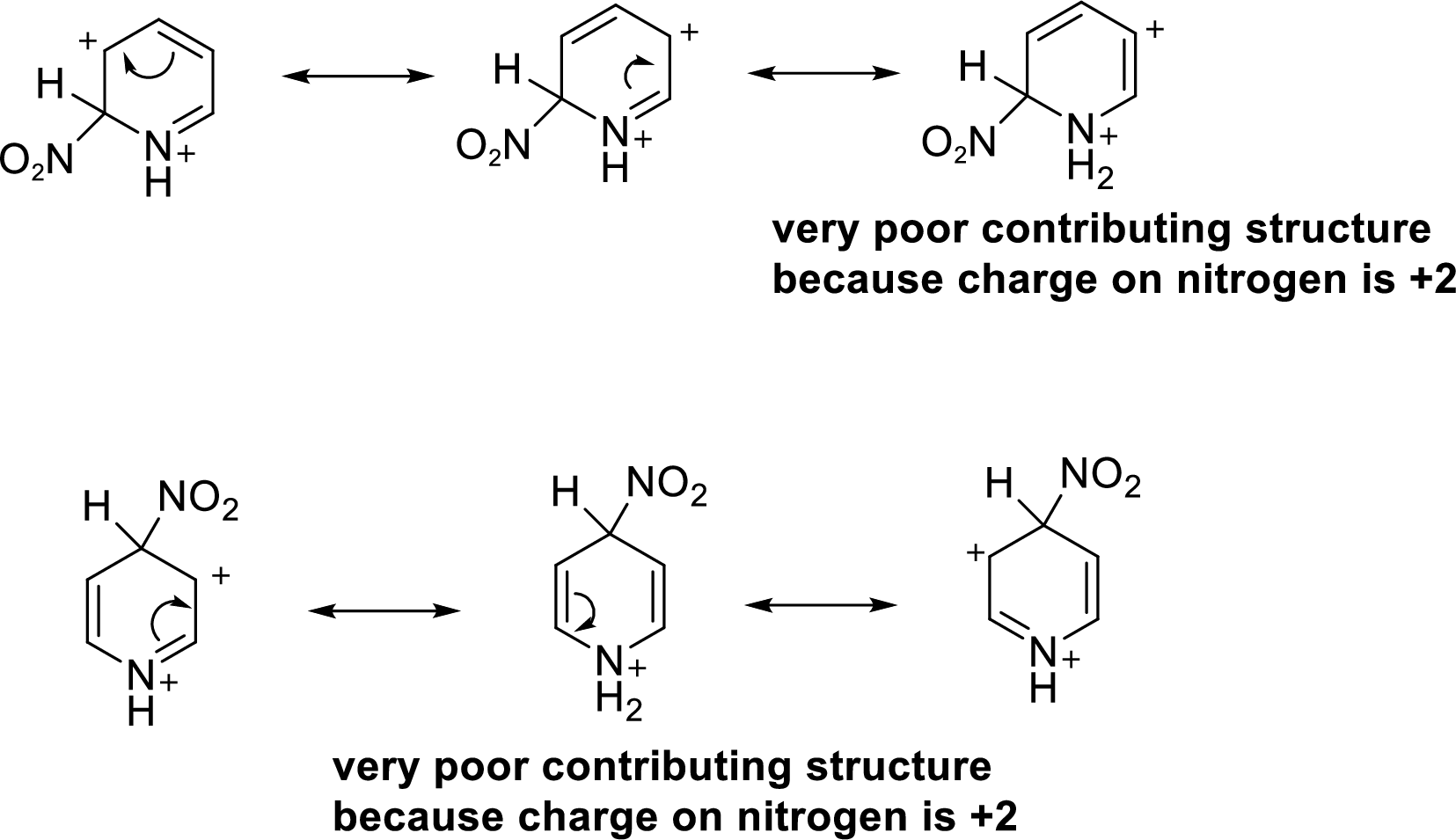
For nitration at the 2nd position or 4th position, the additional positive charge on the cation intermediate is also delocalized on three atoms of the pyridine ring, but one of the resonance structure has a +2 charge on nitrogen atom. This situation is less stable than that happens for nitration at the 3rd position.
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry
- To synthesize trans-cinnamic acid from benzaldehyde (PhCHO) and acetic anhydride (Ac2O) under basic, refluxing conditions to exemplify the Perkin condensation. 1. Draw the complete chemical reaction to be carried out. 2. Provide a complete arrow-pushing mechanism for the synthesis of trans-cinnamic acid. 3. Briefly describe the role of water, Na2CO3, and HCl in the isolation of the product. Draw the structure of the product at basic pH (after Na2CO3 addition) and at acidic pH (after HCl addition).arrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.)p-Chlorobenzoic acidarrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.)m-Nitrobenzenesulfonic acidarrow_forward
- (17, 18, 5) Give the relative rates of reaction of the four carboxylic acid derivatives below with aqueous sodium hydroxide to give the sodium carboxylate salt.arrow_forwardThe base-promoted rearrangement of an -haloketone to a carboxylic acid, known as the Favorskii rearrangement, is illustrated by the conversion of 2-chlorocyclohexanone to cyclopentanecarboxylic acid. It is proposed that NaOH first converts the a-haloketone to the substituted cyclopropanone shown in brackets and then to the sodium salt of cyclopentanecarboxylic acid. (a) Propose a mechanism for base-promoted conversion of 2-chlorocyclohexanone to the proposed intermediate. (b) Propose a mechanism for base-promoted conversion of the proposed intermediate to sodium cyclopentanecarboxylate.arrow_forward5. Give the structures of the following acids and arrange them in the increasing order of acidity. Give reason.2-Chlorobutanoic acid, 4–chlorobutanoic acid, 3-chloropropanoic acid, 2-chloro propanoic acid and 4-cyanobutanoic acid.arrow_forward
- Give an explanation, why the acidity of these phenolic derivatives give these pKa value? Describe it by using the electronic effect on the acidity.arrow_forwardWrite the equilibrium-constant expressions and obtainnumerical values for each constant in. (a) the basic dissociation of aniline, C6H5NH2. (b) the acidic dissociation of hypochlorous acid,HClO. (c) the acidic dissociation of methyl ammoniumhydrochloride, CH3NH3Cl. (d) the basic dissociation of NaNO2. (e) the dissociation of H3AsO3to H3O+and AsO33-. (f) the reaction of C2O42-with H2O to give H2C2O4and OH-. show solutionarrow_forwardStarting with benzene, toluene, or phenol as the only sources of aromatic rings, show how to synthesize the following. Assume in all syntheses that mixtures of ortho-para products can be separated into the desired isomer. Q.) m-Chlorobenzoic acidarrow_forward
- Each of the following reactions has been carried out under conditions such that disubstitution or trisubstitution occurred. Identify the principal organic product in each case. (a) Nitration of p-chlorobenzoic acid (dinitration) (b) Bromination of aniline (tribromination) (c) Bromination of o-aminoacetophenone (dibromination) (d) Bromination of p-nitrophenol (dibromination) (e) Reaction of biphenyl with tert-butyl chloride and iron(III) chloride (dialkylation) (f) Sulfonation of phenol (disulfonation)arrow_forwardExplain how benzaldehyde and dimedone reacts with each other, and then with the aminotriazole to form compound 1a in the presence of an acid catalyst. Provide a detailed reaction mechanism. During the development of the optimized procedure for the experiment, it was found out that compound 1b can also be produced from the same set of starting materials. Propose a detailed reaction mechanism for the formation of 1b. Explain your answer. What factor/s may drive the formation of 1b over 1a?arrow_forward(a) Write the structures of main products when benzene diazonium chloride reacts with the following reagents :(i) H3PO2 + H2O (ii) CuCN/KCN (iii) H2O(b) Arrange the following in the increasing order of their basic character in an aqueous solution :C2H5NH2, (C2H5)2NH, (C3H5)3N(c) Give a simple chemical test to distinguish between the following pair of compounds :C6H5—NH2 and C6H5—NH—CH3arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
