
a)
Interpretation:
The steps involved in preparing the compound represented by the model, using either malonic ester synthesis or an acetoacetic ester synthesis, are to be given.
Concept introduction:
Acetoacetic ester synthesis converts an
Both reactions involve the same steps such as i) enolate ion formation ii) SN2 attack of the enolate anion on the alkyl halide iii) hydrolysis and decarboxylation.
Answer to Problem 17VC
The steps involved in preparing the compound represented by the model using acetoacetic ester synthesis are given below.
Explanation of Solution
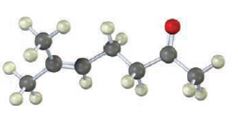
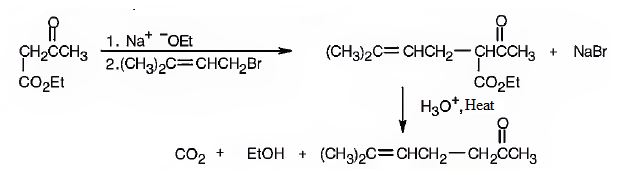
The compound represented by the model is 6-methylhept-5-ene-2-one. It is a methyl ketone and hence it can be prepared using acetoacetic ester synthesis. The ethoxide ion abstracts a proton from the active methylene group of the ester to form the enolate ion. The enolate ion then attacks 1-bromo-3-methy-2-butene and displaces the bromine as bromide ion. The product obtained upon hydrolysis with dilute acids and decarboxylation by heating yields the product.
The steps involved in preparing the compound represented by the model using acetoacetic ester synthesis are given below.

b)
Interpretation:
The steps involved in preparing the compound represented by the model using either malonic ester synthesis or an acetoacetic ester synthesis are to be given.
Concept introduction:
Acetoacetic ester synthesis converts an alkyl halide in to a methyl ketone having three more carbons. The methyl ketone part comes from acetoacetic eater while the remaining carbon comes from the primary alkyl halide. Malonic ester synthesis converts an alkyl halide to a carboxylic acid having two more carbon atoms.
Both reactions involve the same steps such as i) enolate ion formation ii) SN2 attack of the enolate anion on the alkyl halide iii) hydrolysis and decarboxylation.
Answer to Problem 17VC
The steps involved in preparing the compound represented by the model using malonic ester synthesis are given below.
Explanation of Solution
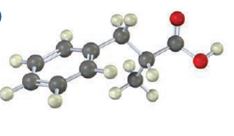
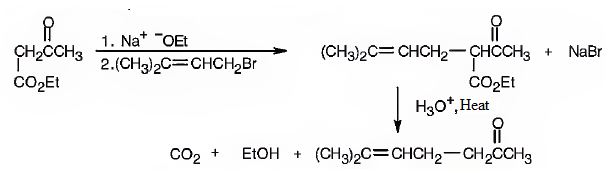
The compound represented by the model is 2-methyl-3-phenylpropanoic acid and hence it can be prepared using malonic ester synthesis. The ethoxide ion abstracts a proton from the active methylene group to form the enolate ion. The enolate ion then attacks benzyl bromide and displaces the bromine as bromide ion. The abstraction of another acidic hydrogen in the product by the base and the nucleophilic displacement of bromine from methyl bromide by enolate ion introduces a methyl group at α- position of the diester. The alkylated diester obtained upon hydrolysis with aqueous acids and decarboxylation by heating yields the product.
The steps involved in preparing the compound represented by the model using malonic ester synthesis are given below.

Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry
- Show how the following compound can be prepared by a malonic or acetoacetic ester synthesis (or other B-dicarbonyl) reaction:arrow_forwardPropose an efficient synthesis for the following transformations using ONLY malonic ester synthesis.arrow_forwardShow how to synthesize the following compound using either the malonic ester synthesis or the acetoacetic ester synthesis. Q.) Cyclopropanecarboxylic acidarrow_forward
- If methanol rather than water is added at the end of a Hell-Volhard-Zelinskii reaction, an ester rather than an acid is produced. Show how you would carry out the following transformation, and propose a mechanism for the ester-forming step.arrow_forwardPropose a reaction for the formation of the following products involving esterformation.arrow_forwardHow would you synthesize the methyl ketone shown below via the acetoacetic ester synthesis? You must show the reactions/reagents used in their correct orderarrow_forward
- Show how to synthesize the following compound using either the malonic ester synthesis or the acetoacetic ester synthesis. Q.) Cyclobutyl methyl ketonearrow_forwardShow how the following compounds can be made using the malonic ester synthesis.(a) 3-phenylpropanoic acidarrow_forwardPropose an efficient synthesis for the following transformations using ONLY acetoacetic ester synthesis. R1 and R2 are the alkyl groups that should be added to the alpha carbon labeled in one of the attached image.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

