
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22.SE, Problem 37AP
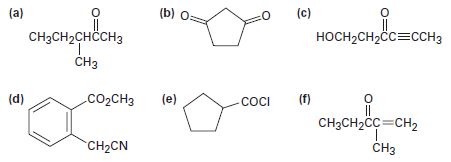
Identify all the acidic hydrogens (pKa < 25) in the following molecules:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
please provide the machanisms of 1a, 1e, 1f
The mass spectrum of the following compound shows fragments at m/z= 127, 113, and 85. Propose structures for the ions that give rise to thesepeaks.
The leaves of the Brazilian Tree Senna multijunga contain a number of pryidine alkaloids that inhibit acetylcholinterinase. Two recentyl isolated isomeric compounds have the strcture have the strcture shown below. (NOTE: M=293) Use the mass spectral data provided to determine the precise location of the hydroxyl group in each isomer.
Isomer A: EI-MS, m/z(rel. int): 222(20), 150(10), 136(25), 123(100)
Isomer B:EI-MS, m/z(re;. int): 236(20), 150(10), 136(25), 123(100)
Chapter 22 Solutions
Organic Chemistry
Ch. 22.1 - Prob. 1PCh. 22.1 - How many acidic hydrogens does each of the...Ch. 22.1 - Prob. 3PCh. 22.3 - Write the complete mechanism for the deuteration...Ch. 22.3 - Prob. 5PCh. 22.4 - If methanol rather than water is added at the end...Ch. 22.5 - Prob. 7PCh. 22.5 - Draw a resonance structure of the acetonitrile...Ch. 22.6 - If methanol rather than water is added at the end...Ch. 22.7 - Prob. 10P
Ch. 22.7 - Draw a resonance structure of the acetonitrile...Ch. 22.7 - Why do you suppose ketone halogenations in acidic...Ch. 22.7 - Prob. 13PCh. 22.7 - Prob. 14PCh. 22.7 - Prob. 15PCh. 22.7 - Prob. 16PCh. 22.SE - Prob. 17VCCh. 22.SE - Prob. 18VCCh. 22.SE - Prob. 19VCCh. 22.SE - Prob. 20MPCh. 22.SE - Predict the product(s) and provide the mechanism...Ch. 22.SE - Predict the product(s) and provide the mechanism...Ch. 22.SE - Prob. 23MPCh. 22.SE - In the Hell–Volhard–Zelinskii reaction, only a...Ch. 22.SE - Prob. 25MPCh. 22.SE - Nonconjugated , -unsaturated ketones, such as...Ch. 22.SE - Prob. 27MPCh. 22.SE - Using curved arrows, propose a mechanism for the...Ch. 22.SE - Prob. 29MPCh. 22.SE - One of the later steps in glucose biosynthesis is...Ch. 22.SE - The Favorskii reaction involves treatment of an...Ch. 22.SE - Treatment of a cyclic ketone with diazomethane is...Ch. 22.SE - Prob. 33MPCh. 22.SE - Amino acids can be prepared by reaction of alkyl...Ch. 22.SE - Amino acids can also be prepared by a two-step...Ch. 22.SE - Heating carvone with aqueous sulfuric acid...Ch. 22.SE - Identify all the acidic hydrogens (pKa 25) in the...Ch. 22.SE - Rank the following compounds in order of...Ch. 22.SE - Prob. 39APCh. 22.SE - Base treatment of the following , -unsaturated...Ch. 22.SE - Prob. 41APCh. 22.SE - Prob. 42APCh. 22.SE - Prob. 43APCh. 22.SE - Which, if any, of the following compounds can be...Ch. 22.SE - Prob. 45APCh. 22.SE - Prob. 46APCh. 22.SE - Prob. 47APCh. 22.SE - How might you convert geraniol into either ethyl...Ch. 22.SE - Prob. 49APCh. 22.SE - One way to determine the number of acidic...Ch. 22.SE - Prob. 51APCh. 22.SE - Prob. 52APCh. 22.SE - Prob. 53APCh. 22.SE - Prob. 54APCh. 22.SE - Prob. 55APCh. 22.SE - Prob. 56APCh. 22.SE - All attempts to isolate primary and secondary...Ch. 22.SE - How would you synthesize the following compounds...Ch. 22.SE - Prob. 59APCh. 22.SE - Prob. 60APCh. 22.SE - Prob. 61APCh. 22.SE - Prob. 62APCh. 22.SE - As far back as the 16th century, South American...Ch. 22.SE - The key step in a reported laboratory synthesis of...Ch. 22.SE - Prob. 65AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Propose structures for compounds that fit the following mass-spectral data: (a) A hydrocarbon with M+=132 (b) A hydrocarbon with M+=166 (c) A hydrocarbon with M+=84arrow_forwardClCH₂CO₂H , elucidate the following mass spectra and give atleast 5 fragmentationsarrow_forwardThe mass spectrum of 2,3-dimethylpentane [(CH3)2CHCH(CH3)CH2CH3]shows fragments at m/z = 85 and 71. Propose possible structures for theions that give rise to these peaks.arrow_forward
- ClCH₂CO₂H , elucidate the following spectra bellowarrow_forwardSHOW COMPLETE SOLUTIONS Q3. Which isolate has A260/280 ratio of above 2.0?arrow_forwardHelp needed with the following C-NMR analysis on the molecule hexaphenylbenzene. Note: we can assume the ispo carbons for the central ring are downfield of those of the subtitients. "Ipso" standing for the aromatic carbon to which a susbstituent is attached. Thank you in advance!arrow_forward
- 3b. 2-Methylhexane shows an intense peak in the mass spectrum at m/z = 85, 57 and 43. Propose the possible structures for these fragments.arrow_forwardThe mass spectrum of 1-ethyl-1-methylcyclohexane shows many fragments, with two in very large abundance. Kne appears af m/z=111 and the other appears at m/z=97. Identify the structure of each of these fragments.arrow_forwardWhich amomg the fragments below will be dectected by mass Spectrophotometer? [CH3CH3]+ CH3CH3 •CH2CH3 [CH3CH4]- only ii i and iii only i ii and iv only iiarrow_forward
- Answer Q35, 34 showing detailly all explanationsarrow_forwardAttached is the structure and nmr of [(+)Co(en)3]I3 * H2O . Please assign placement for the non equivalent hydrogen atoms and integrate. .arrow_forwardORGO II NMR please help fill out the chart and label. my unknown is C6H10O.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY