
Concept explainers
(a)
Interpretation: The theoretical yield of
Concept introduction:
Number of moles of a substance,
From its given mass is,
Theoretical yield is the maximum product yield that can be expected based on the masses of the reactants and the reaction stoichiometry.
2-Iodobenzoic acid is a crystaline solid and it can be prepared from 2-aminobenzoic acid treated with
The reaction can be represnted as follows,
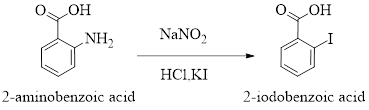
(a)
Answer to Problem 104IL
Theoretical yield of benzoic acid is
Explanation of Solution
From the given data,
Let’s calculate the number of moles of 2-amino benzoic acid:
From the given:
Let’s calculate the number of moles of
From the given:
Let’s calculate the number of moles of
From the equation , we can see that 1 mole of 2- amino benzoic acid gives 1 mole of 1-iodo benzoic acid .
For this conversion required 1 mole of
Theoretically produced
Molar mass of 2-iodobenzoic acid =
Therefore, theoretical yield of 2-iodobenzoic acid is
(b)
Interpretation: To Check the possibilty of isomers for 2-iodobenzoic acid
Concept introduction:
2-Iodobenzoic acid is a crystaline solid and it can be prepared from 2-aminobenzoic acid treated with
The reaction can be represnted as follows,
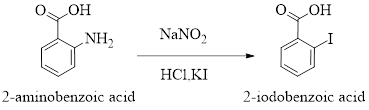
Two compounds that have the same molecular formula, but have different structural formulas are called isomers.
(b)
Answer to Problem 104IL
No other possible isomers for 2-Iodobenzoic acid.
Explanation of Solution
No other isomers of 2-iodo benzoic acid are possible in this reaction because the
(c)
Interpretation: The molar mass of the product formed in the given reaction has to be determined. And check whether the calculated molar mass is in reasonable agreement with the theoretical molar mass or not.
Concept introduction:
2-Iodobenzoic acid is a crystaline solid and it can be prepared from 2-aminobenzoic acid treated with
The reaction can be represnted as follows,
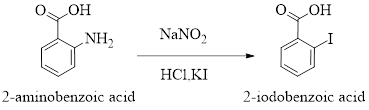
Concentration of solutions can be expressed in various terms; molarity is one such concentration expressing term.
Molarity (M) of a solution is the number of gram moles of a solute present in one liter of the solution.
A solution containing one gram mole or
Amount of substance (mol) can be determined by using the equation,
The molar mass of an element or compound is the mass in grams of 1 mole of that substance, and it is expressed in the unit of grams per mol (g/mol).
Theoretical yield is the maximum product yield that can be expected based on the masses of the reactants and the reaction stoichiometry.
(c)
Answer to Problem 104IL
Molar mass of 2-Iodobenzoic acid
There is only
Explanation of Solution
From the given,
Let’s calculate the number of mole of
Let’s calculate molar mass of 2-iodo benzoic acid.
Theoretical mass 2-Iodobenzoic acid is
Therefore, there is only
We can say that it is in reasonable agreement with theoretical molar mass.
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry & Chemical Reactivity
- Maleic acid and fumaric acid are the cis- and trans- isomers, respectively, of C2H2(COOH)2, a dicarboxylic acid. Draw and label their structures.arrow_forward1. Write the structure of the compound 1-amino-4-nitro naphthalene?arrow_forwardAn isomer of C6H12O could contain a carboxylic acid. True or false?arrow_forward
- 1. Name the common amino acids that contain an aromatic ring. Enter your answers in alphabetical order separated by commas. 2. Name the common amino acids that contain sulfur. Enter your answers separated by commas. 3. Name the common amino acids that are alcohols. Enter your answers separated by commas. 4. Name the common amino acids that have alkyl-group side chains. Enter your answers separated by commas.arrow_forwardHow many grams of salicylic acid should be added to 75 g of polyethylene glycol ointment to prepare an ointment containing 6% w/w of salicylic acid?arrow_forwardGive two tests used in the identification of ethyl acetate? Show the chemical reactions taking place.arrow_forward
- There are two different butanoic acids with the formula C5H10O2. Draw and name them.arrow_forwardTreating chitin with H2O, -OH hydrolyzes its amide linkages, forming a compound called chitosan. What is the structure of chitosan? Chitosan has been used in shampoos, bers for sutures, and wound dressings.arrow_forwardIn 1822, Friedrich Wohler produced the component the compound urea, H2N-CO-NH2 in the labratory. Why was the result of this experiement significant in the history of organic chemistry? and What is the equation showing the synthesis of urea from ammonia cyanate?arrow_forward
- What is a molecule that fits these three criteria: It should have a molar mass between 180 and 280 g/mol, contain a substituted cyclohexane like e. g. 5,6-dihydroxy-bicyclo[2.2.2]octane-2-carboxylic acid methyl ester, and be optically active.arrow_forwardNomex®, a strong fire-resistant fabric, is a polyamide made from meta-phthalic acid and meta-diaminobenzene. Draw the structure of Nomex.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





