
Concept explainers
Interpretation:
The oxygen containing group should be identified for the given molecule.
Concept introduction:
If the compound is having oxygen atom it must be an alcohol
Example is given below
If the molecule is having hydroxyl group is called as an alcohol.
For example:

If the molecule is having (
For example:
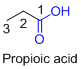
If the molecule is having (
For example
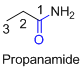
If the molecule is having (
Example
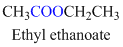
If the molecule is having (
Example

The given compound is (
Example

Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
- encircle and identify the functional groupsarrow_forwardThe fact that sweet-tasting carbohydrates like table sugar are also high in calories has prompted the development of sweet, low-calorie alternatives. (a) Identify the functional groups in aspartame, the artificial sweetener in Equal. (b) Label all of the sites that can hydrogen bond to the oxygen atom of water.arrow_forwardDraw the correct structure for compound.1-heptynearrow_forward
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





