
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN: 9781305580343
Author: Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 23, Problem 23.18QP
What is the correct IUPAC name for the following compound?
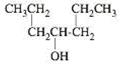
- a 4-heptenol
- b 3-methyl-2-pentanol
- c 4-pentanol
- d 4-heptanol
- e 1,3-diethyl-2-propanol
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
General Chemistry - Standalone book (MindTap Course List)
Ch. 23.2 - Prob. 23.1ECh. 23.2 - In the model shown here, C atoms are black and H...Ch. 23.2 - Prob. 23.2CCCh. 23.3 - Prob. 23.2ECh. 23.3 - Prob. 23.3ECh. 23.5 - Prob. 23.4ECh. 23.5 - Prob. 23.5ECh. 23.5 - Prob. 23.6ECh. 23.5 - Prob. 23.7ECh. 23.5 - Prob. 23.8E
Ch. 23.5 - Give the IUPAC name for each of the following...Ch. 23.5 - Prob. 23.10ECh. 23.5 - Prob. 23.3CCCh. 23.6 - Prob. 23.11ECh. 23.6 - Prob. 23.12ECh. 23.6 - Prob. 23.13ECh. 23 - Give the molecular formula of an alkane with 25...Ch. 23 - Prob. 23.2QPCh. 23 - Prob. 23.3QPCh. 23 - Prob. 23.4QPCh. 23 - Prob. 23.5QPCh. 23 - Prob. 23.6QPCh. 23 - Prob. 23.7QPCh. 23 - Prob. 23.8QPCh. 23 - What would you expect to be the major product when...Ch. 23 - Prob. 23.10QPCh. 23 - Prob. 23.11QPCh. 23 - Prob. 23.12QPCh. 23 - Prob. 23.13QPCh. 23 - Prob. 23.14QPCh. 23 - Prob. 23.15QPCh. 23 - Prob. 23.16QPCh. 23 - Prob. 23.17QPCh. 23 - What is the correct IUPAC name for the following...Ch. 23 - Prob. 23.19QPCh. 23 - Prob. 23.20QPCh. 23 - Explain why you wouldnt expect to find a compound...Ch. 23 - Catalytic cracking is an industrial process used...Ch. 23 - Prob. 23.23QPCh. 23 - In the models shown here, C atoms are black and H...Ch. 23 - Prob. 23.25QPCh. 23 - Prob. 23.26QPCh. 23 - Prob. 23.27QPCh. 23 - Prob. 23.28QPCh. 23 - Prob. 23.29QPCh. 23 - Prob. 23.30QPCh. 23 - Complete and balance the following equations. Note...Ch. 23 - Prob. 23.32QPCh. 23 - Prob. 23.33QPCh. 23 - Prob. 23.34QPCh. 23 - Prob. 23.35QPCh. 23 - Complete the following equation, giving only the...Ch. 23 - Prob. 23.37QPCh. 23 - What is the IUPAC name of each of the following...Ch. 23 - Prob. 23.39QPCh. 23 - Write the condensed structural formula for each of...Ch. 23 - Give the IUPAC name of each of the following. a...Ch. 23 - For each of the following, write the IUPAC name. a...Ch. 23 - Prob. 23.43QPCh. 23 - Prob. 23.44QPCh. 23 - Prob. 23.45QPCh. 23 - Prob. 23.46QPCh. 23 - Give the IUPAC name of each of the following...Ch. 23 - Prob. 23.48QPCh. 23 - Prob. 23.49QPCh. 23 - Prob. 23.50QPCh. 23 - Prob. 23.51QPCh. 23 - Circle and name the functional group in each...Ch. 23 - Prob. 23.53QPCh. 23 - Prob. 23.54QPCh. 23 - Prob. 23.55QPCh. 23 - Prob. 23.56QPCh. 23 - What is the common name of each of the following...Ch. 23 - Prob. 23.58QPCh. 23 - Prob. 23.59QPCh. 23 - Prob. 23.60QPCh. 23 - Prob. 23.61QPCh. 23 - Prob. 23.62QPCh. 23 - Give the IUPAC name of each of the following...Ch. 23 - Prob. 23.64QPCh. 23 - Prob. 23.65QPCh. 23 - Prob. 23.66QPCh. 23 - Prob. 23.67QPCh. 23 - Prob. 23.68QPCh. 23 - Prob. 23.69QPCh. 23 - Prob. 23.70QPCh. 23 - Prob. 23.71QPCh. 23 - A compound with a fragrant odor reacts with dilute...Ch. 23 - Prob. 23.73QPCh. 23 - Prob. 23.74QPCh. 23 - Prob. 23.75QPCh. 23 - Prob. 23.76QPCh. 23 - Prob. 23.77QPCh. 23 - Prob. 23.78QPCh. 23 - Prob. 23.79QPCh. 23 - Prob. 23.80QPCh. 23 - Prob. 23.81QPCh. 23 - Prob. 23.82QPCh. 23 - Prob. 23.83QPCh. 23 - Prob. 23.84QPCh. 23 - Prob. 23.85QPCh. 23 - Prob. 23.86QPCh. 23 - Prob. 23.87QPCh. 23 - Prob. 23.88QP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- List the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forwardDetermine the maximum number of hydrogen bonds that can form between a methanol molecule and a. other methanol molecules b. water molecules c. 1-propanol molecules d. 2-propanol moleculesarrow_forwardWrite a condensed structural formula for each of the following alcohols. a. 2-Methyl-1-propanol b. 4-Methyl-2-pentanol c. 2-Phenyl-2-propanol d. 2-Methylcyclobutanolarrow_forward
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY