
(a) Define carbocation.
(b) Which of the following are carbocations?
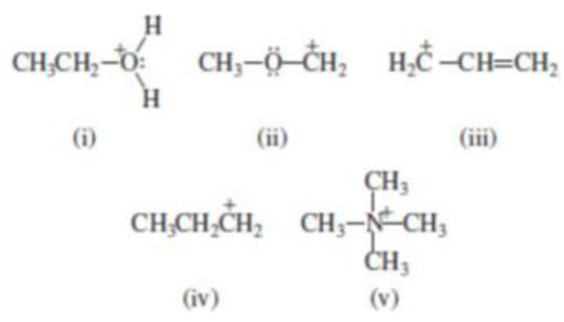
(i)
Interpretation:
Explanation of carbocation, Carbocation should be identified for the given molecule.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon.
Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation.
Answer to Problem 23.53QP
Answer
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation. (a).
Explanation of Solution
To explain:carbocation
Structure of the carbocation is given below,
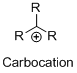
Carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
(ii)
Interpretation:
Explanation of carbocation, Carbocation should be identified for the given molecule.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon.
Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation.
Answer to Problem 23.53QP
Answer
The given molecule is not a carbocation, the structure of the molecule is shown below
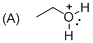
Explanation of Solution
To find: The carbocation.
The given molecule is not a carbocation, the structure of the molecule is shown below
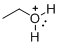
Oxygen is bearing positive charge, so the given molecule is not a carbocation.
(iii)
Interpretation:
Explanation of carbocation, Carbocation should be identified for the given molecule.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon.
Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation.
Answer to Problem 23.53QP
Answer
The given molecule is carbocation, the structure of the molecule is shown below
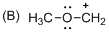
Explanation of Solution
To find: The carbocation.
The given molecule is carbocation, the structure of the molecule is shown below
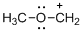
Carbon atom bears positive charged species with three bonds so the given molecule is called Carbocation
(vi)
Interpretation:
Explanation of carbocation, Carbocation should be identified for the given molecule.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon.
Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation.
Answer to Problem 23.53QP
Answer
The given molecule is carbocation, the structure of the molecule is shown below (d)
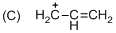
Explanation of Solution
To find:The carbocation.
The given molecule is carbocation, the structure of the molecule is shown below
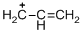
Carbon atom bears positive charged species with three bonds so the given molecule is called Carbocation
(v)
Interpretation:
Explanation of carbocation, Carbocation should be identified for the given molecule.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon.
Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation.
Answer to Problem 23.53QP
Answer
The given molecule is carbocation, the structure of the molecule is shown below (e)
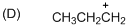
Explanation of Solution
To find:The carbocation.
The given molecule is carbocation, the structure of the molecule is shown below
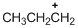
Carbon atom bears positive charged species with three bonds so the given molecule is called Carbocation.
(vi)
Interpretation:
Explanation of carbocation, Carbocation should be identified for the given molecule.
Concept introduction:
Carbocation: carbon atom bears positive charged species with three bonds is called Carbocation and it plays vital intermediate in organic synthesis. Carbocation migratory aptitude is mainly depends on the stability of the carbocation.
Carbocation’s are classified in to three types as primary, secondary and tertiary depending on the number of carbon atoms which is attached to the ionized carbon.
Tertiary carbocation is more stable than secondary carbocation, secondary carbocation is more stable than the primary carbocation.
Answer to Problem 23.53QP
Answer
The given molecule is not a carbocation, the structure of the molecule is shown below (f)
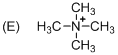
Explanation of Solution
To find: The carbocation.
The given molecule is not a carbocation, the structure of the molecule is shown below (f)
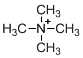
Nitrogen is bearing positive charge with four bonds, so the given molecule is not a carbocation.
Want to see more full solutions like this?
Chapter 23 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
- Disregarding stereoisomers, draw the structures of all alkenes with molecular formula C5H10. Which ones can exist as cis–trans isomers?arrow_forwardA. Which class/classes* of hydrocarbons is/are reactive to Br2 in CH2Cl2 ONLY in the presence of light? b. Which class/classes of hydrocarbons is/are reactive to Br2 in CH2Cl2 WITH OR WITHOUT light? c. Which class/classes of hydrocarbons is/are not reactive to Br2 in CH2Cl2 BOTH in the presence and absence of light?arrow_forwardDraw the condensed structures and give the systematic names for all the alkenes with molecular formula C6H12, ignoring stereoisomers. (Hint: There are 13.) b. Which of the alkenes have E and Z isomers? c. Which of the alkenes is the most stable? d. Which of the alkenes is the least stable?arrow_forward
- When 5-isopropyl-2,3,4,7-tetramethyloctane undergoes complete combustion in the presence of oxygen gas and heat, which of the following substances is/are formed as product/s? Select one or more: Solid carbon Hydrogen gas Water Carbon dioxide Carbon monoxidearrow_forwardWhy is this a cyclohexane ring when there is only one double bond?arrow_forwardIs isomerization of cis and trans -4-methyl-2-pentene possible at room temperature? Explain whyarrow_forward
- Classify the following alcohols as primary, secondary, or tertiary: a. b. CH3CH2CH2CH2OH c.arrow_forwarddraw and name two structures that match the description of a bicyclononanearrow_forwardName all unbranched ether and alcohol isomers with formula C5H12O, and write their structural formulas.arrow_forward
- Viewed through the C-3 — C-4 bond, which is the most stable conformation of 3-bromo-2-methylpentane?arrow_forwarddraw and name two structures that match the description of a trans-dihalocyclopentanearrow_forwardWhich of these alkenes show cis-trans isomerism? For each that does, draw structural formulas for both isomers.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning



