
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
14th Edition
ISBN: 9781259327933
Author: Burdge
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 23, Problem 23.60QP
Consider the following reactions of butanal.
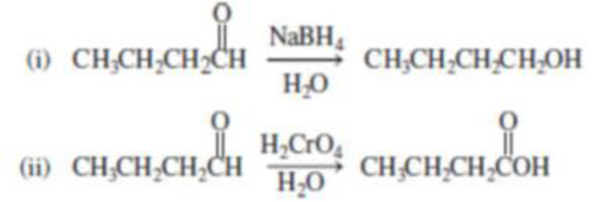
In which reaction is butanal oxidized? In which reaction is it reduced?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Why do bromine water and potassium permanganate not react with benzene? Will cyclohexene react with bromine and potassium permanganate? If so, give the chemical equation.
Which isomers contain an isopropyl group?
a) Draw the structure for ethyl 3-methyl butanoate.
b) Which functional group(s) does this molecule contain?
c) Which type of reaction could be used to synthesize this molecule?
Chapter 23 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
Ch. 23.2 - Prob. 23.1WECh. 23.2 - Prob. 1PPACh. 23.2 - Prob. 1PPBCh. 23.2 - Prob. 1PPCCh. 23.2 - Prob. 23.2WECh. 23.2 - Give the systematic IUPAC name for each of the...Ch. 23.2 - Prob. 2PPBCh. 23.2 - Prob. 2PPCCh. 23.2 - Prob. 23.2.1SRCh. 23.2 - Prob. 23.2.2SR
Ch. 23.2 - Prob. 23.2.3SRCh. 23.2 - Prob. 23.2.4SRCh. 23.2 - Prob. 23.2.5SRCh. 23.2 - Prob. 23.2.6SRCh. 23.3 - Prob. 23.3WECh. 23.3 - Prob. 3PPACh. 23.3 - Prob. 3PPBCh. 23.3 - Prob. 3PPCCh. 23.3 - Prob. 23.4WECh. 23.3 - Prob. 4PPACh. 23.3 - Prob. 4PPBCh. 23.3 - Prob. 4PPCCh. 23.3 - Prob. 23.3.1SRCh. 23.3 - Prob. 23.3.2SRCh. 23.3 - Which of the following pairs of species are...Ch. 23.3 - Prob. 23.3.4SRCh. 23.5 - Prob. 23.5WECh. 23.5 - Prob. 5PPACh. 23.5 - Prob. 5PPBCh. 23.5 - Prob. 23.5.1SRCh. 23.5 - Prob. 23.5.2SRCh. 23 - Prob. 23.1QPCh. 23 - Prob. 23.2QPCh. 23 - Prob. 23.3QPCh. 23 - Prob. 23.4QPCh. 23 - Prob. 23.5QPCh. 23 - Prob. 23.6QPCh. 23 - Prob. 23.7QPCh. 23 - Prob. 23.8QPCh. 23 - Prob. 23.9QPCh. 23 - Name each of the following compounds.Ch. 23 - Prob. 23.11QPCh. 23 - Prob. 23.12QPCh. 23 - Prob. 23.13QPCh. 23 - Prob. 23.14QPCh. 23 - Prob. 23.15QPCh. 23 - Prob. 23.16QPCh. 23 - Prob. 23.17QPCh. 23 - Prob. 23.18QPCh. 23 - Prob. 23.19QPCh. 23 - Prob. 23.20QPCh. 23 - Prob. 23.21QPCh. 23 - Prob. 23.22QPCh. 23 - Prob. 23.23QPCh. 23 - Prob. 23.24QPCh. 23 - Prob. 23.25QPCh. 23 - Prob. 23.26QPCh. 23 - Prob. 23.27QPCh. 23 - Prob. 23.28QPCh. 23 - Prob. 23.29QPCh. 23 - Prob. 23.30QPCh. 23 - Prob. 23.31QPCh. 23 - Prob. 23.32QPCh. 23 - Prob. 23.33QPCh. 23 - Prob. 23.34QPCh. 23 - Fill in the blanks in the given paragraph with the...Ch. 23 - Prob. 23.36QPCh. 23 - Draw all possible structural isomers for the...Ch. 23 - Prob. 23.38QPCh. 23 - Prob. 23.39QPCh. 23 - Prob. 23.40QPCh. 23 - Prob. 23.41QPCh. 23 - Prob. 23.42QPCh. 23 - Prob. 23.43QPCh. 23 - Prob. 23.44QPCh. 23 - Prob. 23.45QPCh. 23 - Prob. 23.46QPCh. 23 - Prob. 23.47QPCh. 23 - Prob. 23.48QPCh. 23 - Prob. 23.49QPCh. 23 - Prob. 23.50QPCh. 23 - Prob. 23.51QPCh. 23 - Prob. 23.52QPCh. 23 - (a) Define carbocation. (b) Which of the following...Ch. 23 - Prob. 23.54QPCh. 23 - Prob. 23.55QPCh. 23 - Prob. 23.56QPCh. 23 - Prob. 23.57QPCh. 23 - Prob. 23.58QPCh. 23 - Prob. 23.59QPCh. 23 - Consider the following reactions of butanal. In...Ch. 23 - Prob. 23.61QPCh. 23 - Prob. 23.62QPCh. 23 - Prob. 23.63QPCh. 23 - Prob. 23.64QPCh. 23 - Prob. 23.65QPCh. 23 - Prob. 23.66QPCh. 23 - Prob. 23.67QPCh. 23 - Prob. 23.68QPCh. 23 - Prob. 23.69QPCh. 23 - Prob. 23.70QPCh. 23 - Prob. 23.71QPCh. 23 - Prob. 23.72QPCh. 23 - Prob. 23.73QPCh. 23 - Prob. 23.74QPCh. 23 - Prob. 23.75QPCh. 23 - Prob. 23.76QPCh. 23 - Prob. 23.77QPCh. 23 - Prob. 23.78QPCh. 23 - Prob. 23.79QPCh. 23 - Prob. 23.80QPCh. 23 - Prob. 23.81QPCh. 23 - Prob. 23.82QPCh. 23 - Prob. 23.83QPCh. 23 - Prob. 23.84QPCh. 23 - Prob. 23.85QPCh. 23 - Prob. 23.86QPCh. 23 - Prob. 23.87QPCh. 23 - Prob. 23.88QPCh. 23 - Prob. 23.89QPCh. 23 - Prob. 23.90QPCh. 23 - Prob. 23.91QPCh. 23 - Prob. 23.92QPCh. 23 - Prob. 23.93QPCh. 23 - Prob. 23.94QPCh. 23 - Prob. 23.95QPCh. 23 - Prob. 23.96QPCh. 23 - Prob. 23.97QPCh. 23 - Prob. 23.98QPCh. 23 - Prob. 23.99QPCh. 23 - Prob. 23.100QPCh. 23 - Prob. 23.101QPCh. 23 - Prob. 23.102QPCh. 23 - Prob. 23.103QPCh. 23 - Prob. 23.104QPCh. 23 - Prob. 23.105QP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Give the molecular formula of a hydrocarbon containing six carbon atoms that is an aromatic hydrocarbon.arrow_forwardCondensed structural formula for methylcylohexane draw and name four isomers of octane and also what can you use to wash the cloth contamined by oil?arrow_forwardA semi-truck loaded with cyclohexane overturns during a rainstorm, spilling its contents over the road embankment. If the rain continues, what will be the fate of the cyclohexane?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY