
Concept explainers
(a)
Interpretation: To determine the number of NADH molecules formed during one turn of the citric acid cycle.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
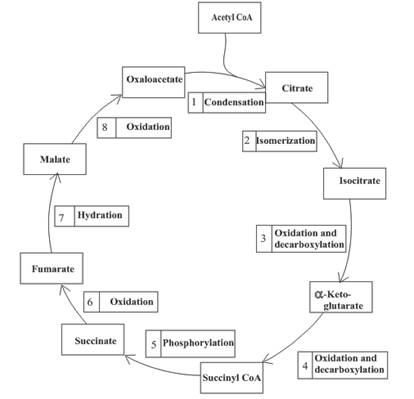
(a)
Answer to Problem 23.70EP
Three molecules of NADH are formed in step 3, 4 and 8 of the citric acid cycle.
Explanation of Solution
Step 3 is the first step where both the oxidation and decarboxylation occurs. Step 3 involves the oxidation of isocitrate and formation of CO2. In this step, firstly isocitrate is oxidized by
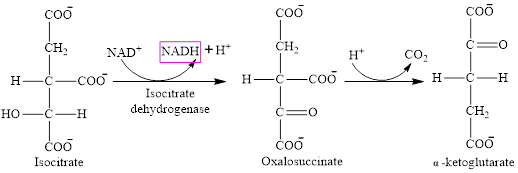
Step 4 involves the oxidation of

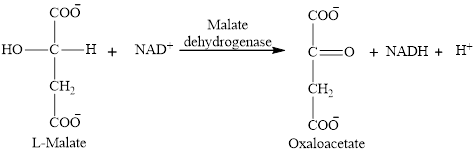
Step 8 is an oxidation reaction and the last step of the citric acid cycle. In step 8,
The reaction of step 8 is:
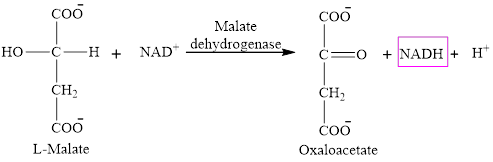
Hence, three molecules of NADH are formed in the citric acid cycle.
(b)
Interpretation: To determine the number of GTP molecules formed during one turn of the citric acid cycle.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
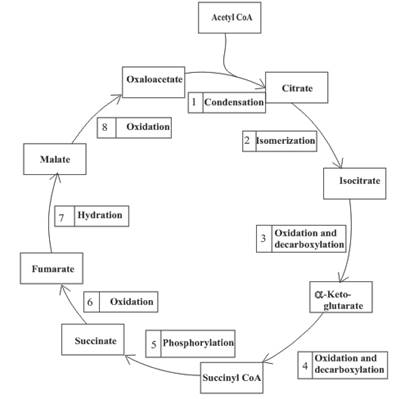
(b)
Answer to Problem 23.70EP
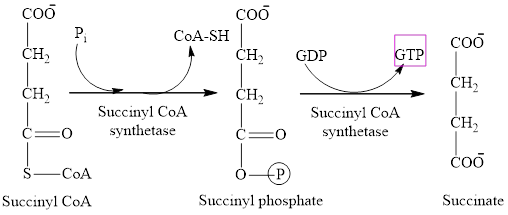
One molecule of GTP is formed in step 5 of the citric acid cycle.
Explanation of Solution
Step 5 involves the thioester bond cleavage in
(c)
Interpretation: To determine the number of time decarboxylation reactions occur during one turn of the citric acid cycle.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
(c)
Answer to Problem 23.70EP
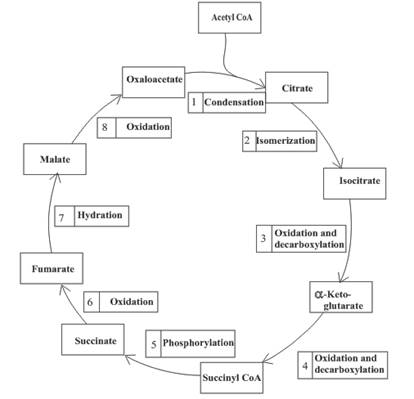
Decarboxylation occurs twice in the citric acid cycle in step 3 and 4.
Explanation of Solution
Step 3 is the first step where both the oxidation and decarboxylation occurs. Step 3 involves the oxidation of isocitrate and formation of CO2. In this step, firstly isocitrate is oxidized by
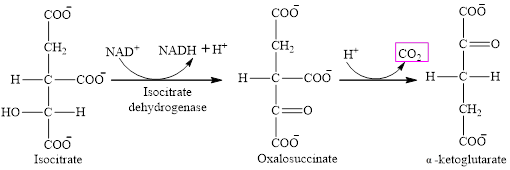
The final product is
Step 4 involves the oxidation of

The final product is
(d)
Interpretation: To determine the number of time oxidation-reduction reaction occur during one turn of the citric acid cycle.
Concept introduction: Citric acid cycle is the third stage of the biochemical energy production process. The cycle includes the reactions in which the acetyl part of acetyl CoA is oxidized and leads to the formation of carbon dioxide and
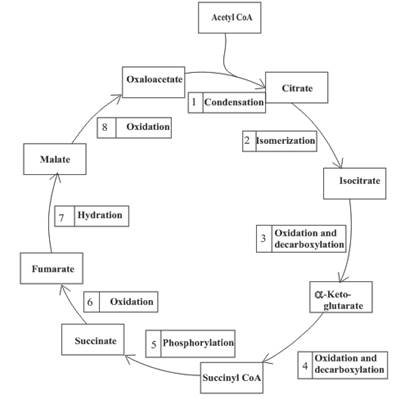
An overview of the citric acid cycle is as follows:
(d)
Answer to Problem 23.70EP
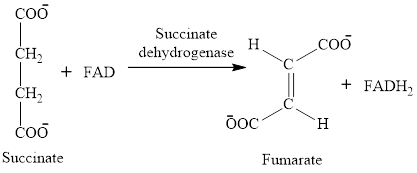
The oxidation-reduction reaction occurs four times in the citric acid cycle in step 3, 4, 6 and 8.
Explanation of Solution
Step 3 is the first step where both the oxidation and decarboxylation occurs. Step 3 involves the oxidation of isocitrate and formation of CO2. In this step, firstly isocitrate is oxidized by
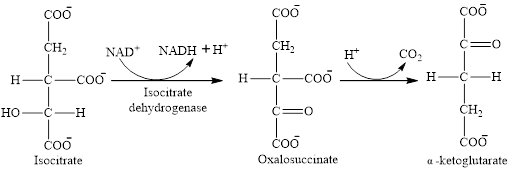
Step 4 involves the oxidation of

In step 6, oxidation of succinate occurs to form fumarate. The enzyme involved in this step of the citric acid cycle is succinate dehydrogenase. FAD is the oxidizing agent in this step. This reaction takes place in the inner mitochondrial membrane. The reaction of step 6 is:
Step 8 is an oxidation reaction and the last step of the citric acid cycle. In step 8,
The reaction of step 8 is:
Want to see more full solutions like this?
Chapter 23 Solutions
General, Organic, and Biological Chemistry
- Which reaction links glycolysis and the citric acid cycle?arrow_forwardIdentify by number those steps of the citric acid cycle that are not redox reactions.arrow_forwardAlthough catabolism of a glucose molecule eventually produces a lot of energy, the first step uses energy. Explain why this step is necessaryarrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


