
Concept explainers
Indicate whether each of the following changes represents oxidation or reduction.
- a. CoQH2 → CoQ
- b. NAD+ → NADH
- c. Cyt c (Fe2+) → cyt c (Fe3+)
- d. Cyt b (Fe3+) → cyt b (Fe2+)
(a)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
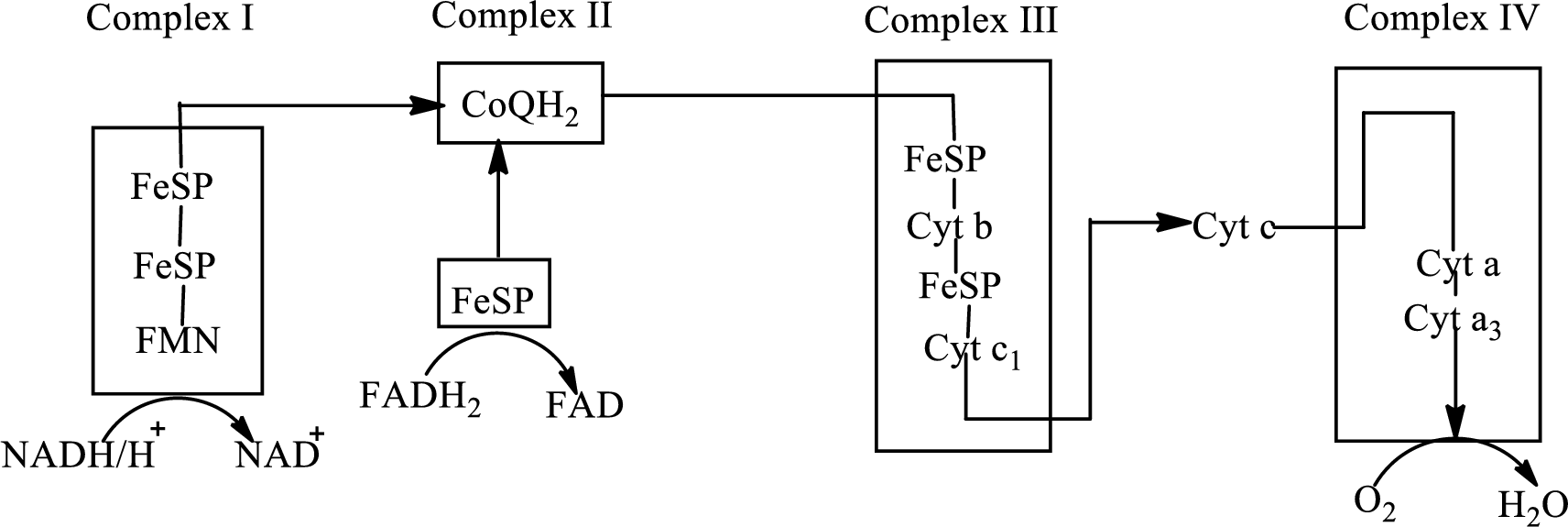
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of the redox reaction is,
Here A is oxidized form and AH is reduced form.
Answer to Problem 23.89EP
The change
Explanation of Solution
(b)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
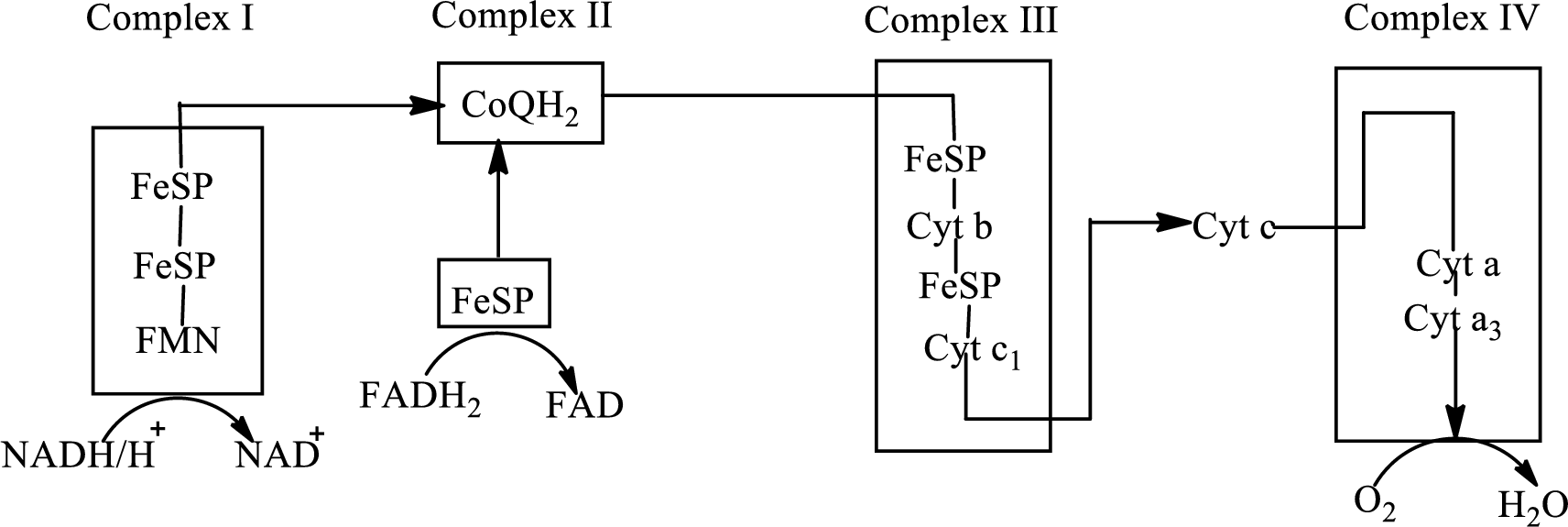
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of the redox reaction is,
Here A is oxidized form and AH is reduced form.
Answer to Problem 23.89EP
The change
Explanation of Solution
In complex I, electrons are transferred from the
The change of
(c)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
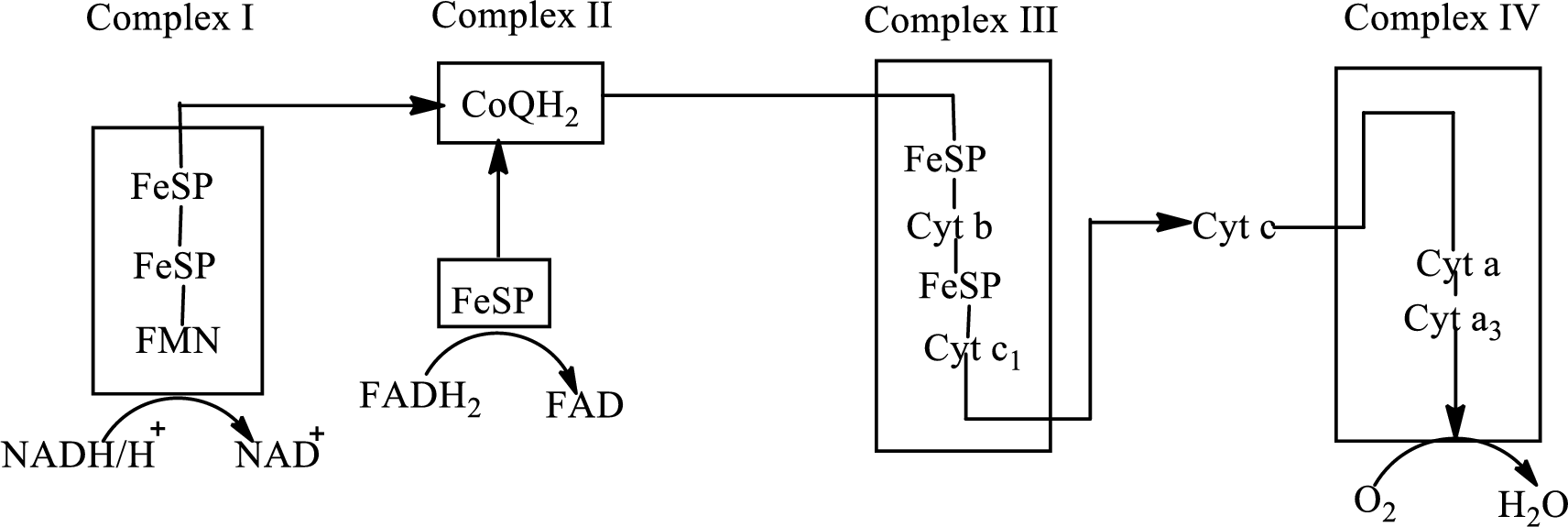
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of the redox reaction is,
Here A is oxidized form and AH is reduced form.
Answer to Problem 23.89EP
The change
Explanation of Solution
(d)
Interpretation:
Whether the change
Concept Introduction:
Electron transport chain is a sequence of biochemical reactions in which electrons and hydrogen atoms from the citric acid cycle are transferred to various intermediate carriers and finally reacts with molecular oxygen to form a water molecule.
There are four complexes associated with the electron transport chain that is present in the inner mitochondrial membrane. The four complexes that help in the electron transfer in the electron transport chain are:
Complex I:
Complex II:
Complex III:
Complex IV:
An overview of the electron transport chain is as follows:
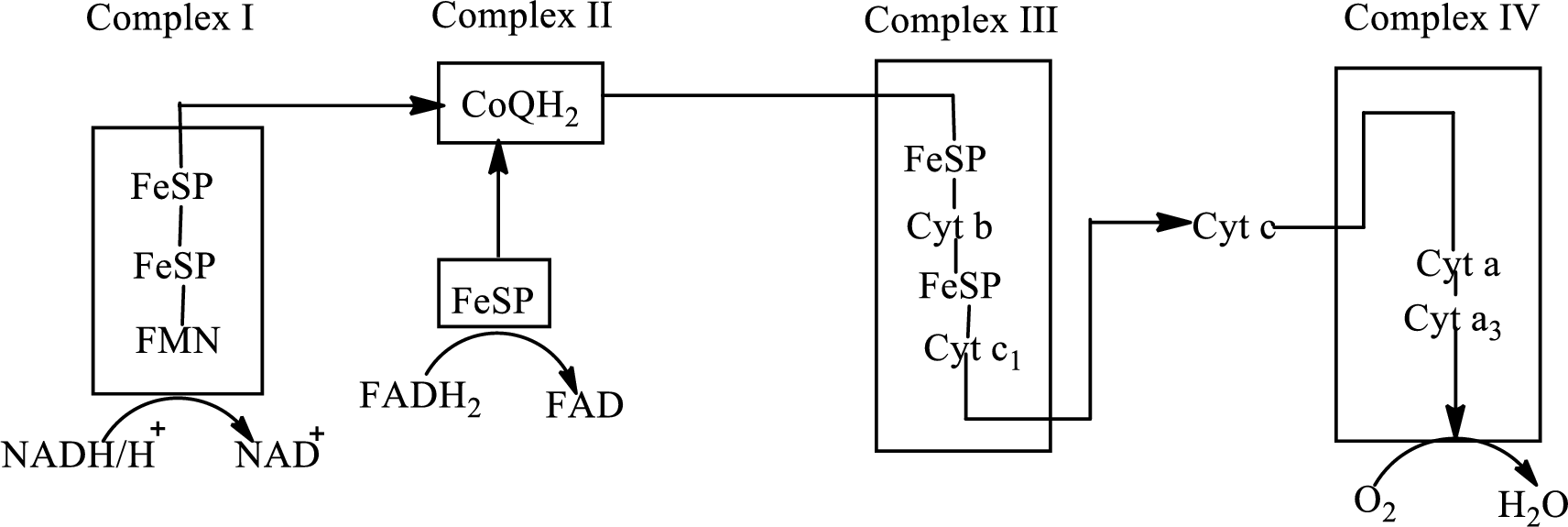
Redox reactions involve oxidation and reduction reaction occurring simultaneously so that one species is oxidized and the other one is reduced. The species that gain hydrogen or electron is known as reduced form and the species that loss hydrogen or electron is known as oxidized form. The general representation of the redox reaction is,
Here A is oxidized form and AH is reduced form.
Answer to Problem 23.89EP
The change
Explanation of Solution
Want to see more full solutions like this?
Chapter 23 Solutions
General, Organic, and Biological Chemistry
- Which of the following can be oxidized to produce ketone: I. Me⁰ alcohol, II. 1⁰ alcohol, III. 2⁰ alcohol, IV. 3⁰ alcohol A.) I and II B.) III only C.) IV only D.) III and IV E.) II, III and IVarrow_forwardA diabetic patient with ketoacidosis has a blood pH of 7.1. Compared to a normal pH of 7.4 this corresponds to what fold-change in hydrogen ion concentration? a. 0.3-fold increase b. 2-fold decrease c. 0.3-fold decrease d. 2-fold increasearrow_forward(1) Slick Johnny burns 1.2 Kcal/min when he's completely chilled and fasted. He lives on moonshine whiskey which is about 40% ethanol (assume a g is about an ml). Assuming there isn't much else of nutritional value, how much would he have to drink to maintain his body weight. 0.62 L 0.82 L 0.93 L 1.44 L (2) Assuming ethanol (C2H6O) is completely burned to CO2 and H2O, what would Slick Johnny's RQ be? 0.67 0.74 0.86 0.96arrow_forward
- Give me a good introduction for the use of citric acid as antioxidant to preserve the flavor of foodsarrow_forwardAfter a redox reaction completes, the electron carrier on the reactant side of the chemical reaction is(reduced or oxidized)while the electron carrier on the product side is( reduced or oxidized ).arrow_forward/ What are Eo' (V) and (kcal/reaction) for the following redox reaction? NADH + H+ + 2 oxidized Cytochrome b NAD+ + 2 reduced Cytochrome b + 2H+arrow_forward
- Remove H2 what will happen to HI?arrow_forwardwhich substance has the greatest entrophy H2O (l) Na(s) AgCL(s) N2(g) NaOH(l)arrow_forward1.) Expenditure of energy that is dependent on body weight, sex, age due to a. basal metabolic rate b. environmental temperature c. physical activity d. thermogenic effect 2.) It is associated with food consumption a. thermogenic effect b. basal metabolic rate c. Physical activity d. environmental temperature 3.) It's our daily energy expenditure a. basal metabolic rate b. environmental temperature c. thermogenic effect d. physical activityarrow_forward
- PCl5 = PCl3 + Cl2 Note: all are gases 1. Balance the equation 2. Tell what will happen to the concentration of Cl2 if: a. Concentration of PCl5 is imcreased B. Pressure is increased c. If concentration of PCl3 is decreased d. If concentration of PCl5 is decreasedarrow_forwardExplain what metabolism, oxidation, and reductionarrow_forwardClassify the following compounds as endergonic or exergonic : (a) glucose,(b) methylamine, (c) octane, (d) ethanol.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning





