
Concept explainers
(a)
Interpretation: The result of treating compound 1 with
Concept introduction:
Reduction reaction: The reaction in which addition of hydrogen takes place is known as reduction reaction.
The reduction of a
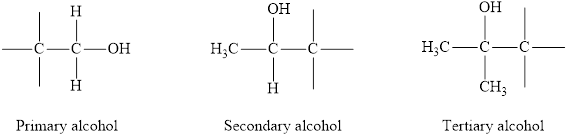
Alcohols are the hydrocarbons which have
Alcohols can be of three types on the basis of degree of carbon atom to which the
Primary alcohol: The
Secondary alcohol: The
Tertiary alcohol: The
(a)
Answer to Problem 62PS
The product obtained from the reaction of given compound
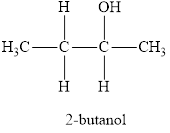
The systematic name of the product is 2-butanol and functional group is alcohol.
Explanation of Solution
Compound 1 s,
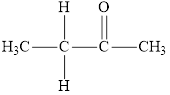
It is a ketone
The reaction between compound
The reduction of a ketone gives a secondary alcohol in presence of
The reaction is in which the compound

The product has same number of carbon atoms as in compound
The parent chain name will be butane and suffix will be “-ol” as there is one
Therefore, name of the product is 2-butanol and functional group is alcohol.
(b)
Interpretation: The structure of the reaction product from comounds 2 and 4 has to be drawn. The functional group of the product has to be identified
Concept introduction:
Ester: One
The name for an ester molecule can be written by using alkyl chain
Esterification reaction: Esters are prepared by the reaction of a
Here, the
(b)
Answer to Problem 62PS
The structure of the product is,
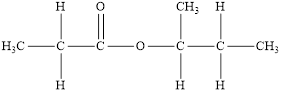
The functional group is an ester
Explanation of Solution
Compound 2 is,
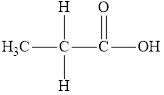
It is a carboxylic acid
Compound 4 is,
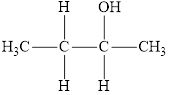
It is a secondary alcohol
Esters are prepared by the reaction of a carboxylic acid and an alcohol molecule with the elimination of water molecule
The reaction is written below in which the compound
Here, the
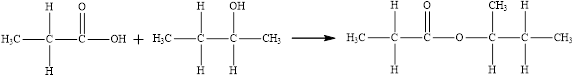
The product has
(c)
Interpretation: The compound that results from the addition of
Concept introduction:
Hydrogenation: The addition of hydrogen to unsaturated compounds to convert into saturated compounds in the presence of catalyst.
An
Alcohols are the hydrocarbons which have
Alcohols can be of three types on the basis of degree of carbon atom to which the
(c)
Answer to Problem 62PS
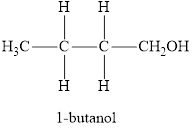
The product obtained from the reaction of given compound
Explanation of Solution
Compound 3 is,
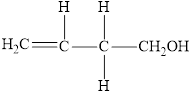
It is an alkene
The reaction between compound
In this reaction, the compound
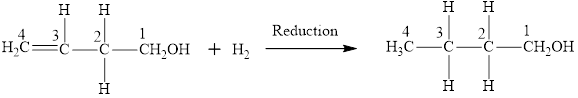
The product has same number of carbon atoms as in compound
The parent chain name will be butane
The suffix will be “-ol” as there is one
Therefore, name of the product is 1-butanol and functional group is alcohol.
(d)
Interpretation: The compound that results from the addition of
Concept introduction:
The reaction of carboxylic acid with
(d)
Answer to Problem 62PS
The product obtained from the reaction of given compound
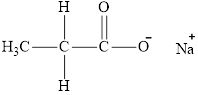
Explanation of Solution
The reaction of carboxylic acid with
The product obtained is the sodium salt of the acid and water.
The reaction of compound
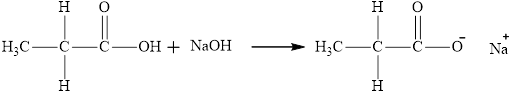
Want to see more full solutions like this?
Chapter 23 Solutions
Chemistry & Chemical Reactivity
- When the conjugate acid of aniline, C6H5NH3+, reacts with the acetate ion, the following reaction takes place: C6H5NH3+(aq)+CH3COO(aq)C6H5NH2(aq)+CH3COOH(aq) If Kafor C6H5NH3+ is 1.35105 and Kafor CH3COOH is 1.86105 , what is K for the reaction?arrow_forward4. Write the formula and name for the product when cyclopentene reacts with (a) Cl2(b) HBr(c) H2, Pt (d) H2O, H+arrow_forwardWhich alcohols can be prepared as a single product by hydroboration– oxidation of an alkene? Which alcohols can be prepared as a single product by the acid-catalyzed addition of H2O to an alkene?arrow_forward
- 7. The following compounds are isomeric esters derived from acetic acid, each with formula C5H10O2. Draw the structures of the two esters?arrow_forward1) The acid-catalyzed dehydration of 2-methyl-2-butanol yields two alkene products, what are the names of the two alkenes? 2) which of the two alkenes is the major product?arrow_forwardwrite the structure formulas of alkanes with molecular formula C6H14, which with chlorine give: a) three monochlorinated isomers? b) five monochlorinated isomers c) only two monochlorinated isomersarrow_forward
- What is the molecular formula of a hydrocarbon with M^+=166? What is the sum of the rings and double bonds in this compound?arrow_forwardDraw 10 structural isomers of C5H10O that contain a ring.arrow_forwardReaction of butane (CH 3CH 2CH 2CH 3) with Cl 2 in the presence of light forms two different alkyl chlorides that have molecular formula C 4H 9Cl. Draw the structures of both alkyl chlorides.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





