
a)
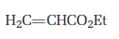
Interpretation:
The product that will be produced when the enamine obtained by reacting cyclopentanone and pyrrolidone with α, β- unsaturated acceptor, ethyl acrylate, is to be identified.
Concept introduction:
The enamine obtained by reacting a
To identify:
The product that will be produced when the enamine obtained by reacting cyclopentanone and pyrrolidone with α, β- unsaturated, ethyl acrylate, is hydrolyzed.
b)
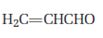
Interpretation:
The product that will be produced when the enamine obtained by reacting cyclopentanone and pyrrolidone with α, β- unsaturated acceptor, acrolein is to be identified.
Concept introduction:
The enamine obtained by reacting a ketone with cyclic amine reacts with α, β- unsaturated acceptor, acrolein, to yield a substituted enamine as the intermediate which upon hydrolysis gives the products.
To identify:
The product that will be produced by the hydrolysis of the enamine obtained by reacting cyclopentanone and pyrrolidone with α, β- unsaturated acceptor, acroline.
c)
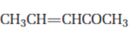
Interpretation:
The product that will be produced when the enamine obtained by reacting cyclopentanone and pyrrolidone with α, β- unsaturated acceptor, pent-3-ene-2-one, is to be identified.
Concept introduction:
The enamine obtained by reacting a ketone with cyclic amine reacts with an α, β- unsaturated acceptor to yield a substituted enamine as the intermediate which upon hydrolysis gives the products.
To identify:
The product that will be produced by the hydrolysis of the enamine obtained by reacting cyclopentanone and pyrrolidone with α, β- unsaturated acceptor, pent-3-ene-2-one.
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Organic Chemistry
- Treatment of 1-aminoadamantane, C10H17N, with methyl 2,4-dibromobutanoate in the presence of a nonnucleophilic base, R3N, involves two successive SN2 reactions and gives compound A. Propose a structural formula for compound A.arrow_forwardShow the products you would obtain by acid-catalyzed reaction of cyclohexanone with ethylamine, CH3CH2NH2, and with diethylamine, (CH3CH2) 2NH.arrow_forwardA step in a synthesis of PGE1 (prostaglandin E1, alprostadil) is the reaction of a trisubstituted cyclohexene with bromine to form a bromolactone. Propose a mechanism for formation of this bromolactone and account for the observed stereochemistry of each substituent on the cyclohexane ring. Alprostadil is used as a temporary therapy for infants born with congenital heart defects that restrict pulmonary blood flow. It brings about dilation of the ductus arteriosus, which in turn increases blood flow in the lungs and blood oxygenation.arrow_forward
- Dihydropyran is synthesized by treating tetrahydrofurfuryl alcohol with an arenesulfonic acid, ArSO3H. Propose a mechanism for this conversion.arrow_forwardWhen cis-2-decalone is dissolved in ether containing a trace of HCl, an equilibrium is established with trans-2-decalone. The latter ketone predominates in the equilibrium mixture. Propose a mechanism for this isomerization and account for the fact that the trans isomer predominates at equilibrium.arrow_forwardThe Meerwein-Ponndorf-Verley reaction involves reduction of a ketone by treatment with an excess of aluminum triisopropoxide, [(CH3)2CHO]3Al. The mechanism of the process is closely related to the Cannizzaro reaction in that a hydride ion acts as a leaving group. Propose a mechanism.arrow_forward
- Compound H (C8H6O3) gives a precipitate when treated with hydroxylamine in aqueous ethanol and a silver mirror when treated with Tollens solution. Following is its 1H-NMR spectrum. Deduce the structure of compound H.arrow_forwardName the following amine, including R, S stereochemistry, and draw the product of its reaction with excess iodomethane followed by heating with Ag2O (Hofmann elimination). Is the stereochemistry of the alkene product Z or E? Explain.arrow_forwardNitriles, R–=C≡N, undergo a hydrolysis reaction when heated with aqueous acid. What is the structure of the compound produced by hydrolysis of propanenitrile, CH3CH2C≡N, if it has IR absorptions from 2500–3100 cm-1 and at 1710 cm-1, and has M+=74?arrow_forward
- 2-Bromo-6, 6-dimethylcyclohexanone gives 2, 2-dimethylcyclopentane- carboxylic acid on treatment with aqueous NaOH followed by acidification, a process called the Favorskii reaction. Propose a mechanism.arrow_forwardAzlactones are important starting materials used in the synthesis of dehydro α-aminoacids. They react with aldehydes to form an intermediate that is hydrolyzed under acidic conditions to give the final amino acid product. Provide the structure of the intermediate and propose a mechanism for its formation.arrow_forwardOne step in the urea cycle for ridding the body of ammonia is the conversion of argininosuccinate to the amino acid arginine plus fumarate. Propose a mechanism for the reaction, and show the structure of arginine.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

