
Concept explainers
a)
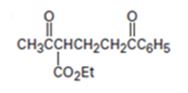
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
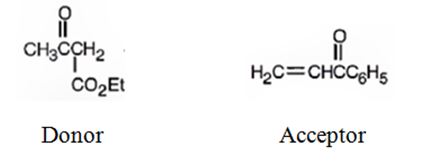
Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between the ethylacetoacetate (nucleophilic donor) and phenyl vinyl ketone (electrophilic acceptor).
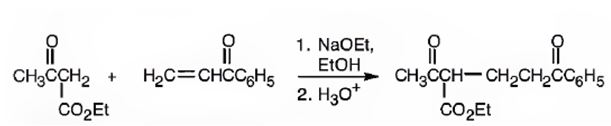
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
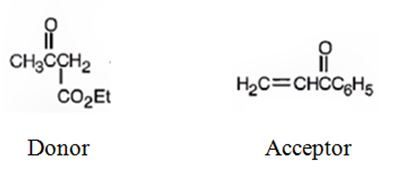
b)
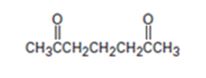
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between the ethylacetoacetate (nucleophilic donor) and methyl vinyl ketone (electrophilic acceptor).
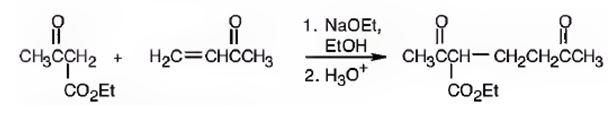
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

c)
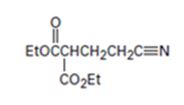
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between the ethylacetoacetate (nucleophilic donor) and vinyl nitrile (electrophilic acceptor).
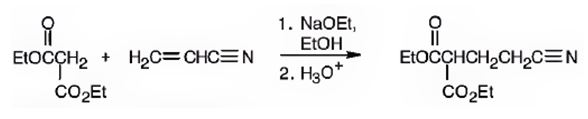
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

d)
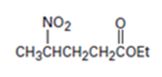
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between nitro ethane (nucleophilic donor) and ethylacrylate (electrophilic acceptor).
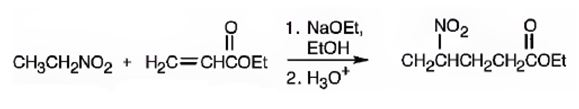
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
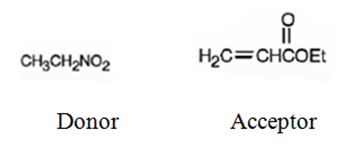
e)
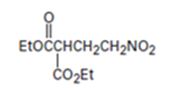
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

Explanation of Solution
An analysis of the structure of the compound indicates that it is formed by the reaction between the ethylsuccinate (nucleophilic donor) and nitro ethene (electrophilic acceptor).
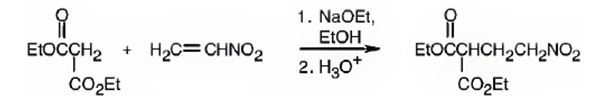
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are

f)
Interpretation:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are to be given.
Concept introduction:
Michael reaction involves the conjugate addition of a stable enolate ion derived from a β-ketoesters or β-diketones or β-ketonitriles or malonic esters (donors) to an unhindered α,β-unsaturated ketones or aldehydes or esters or thioesters or nitriles or amides or nitro compounds (acceptors). The enolate ion from the donor attacks the double bond in acceptor. A new bond is formed between the α-carbon of the donor and the β-carbon of the unsaturated ester.
To give:
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown.
Answer to Problem 62AP
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
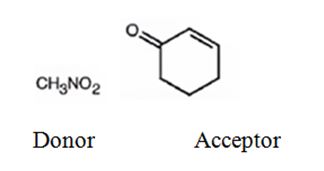
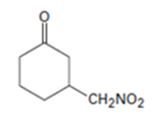
Explanation of Solution
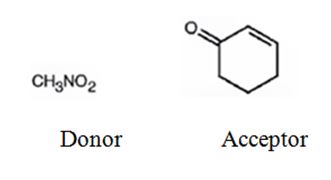
An analysis of the structure of the compound indicates that it is formed by the reaction between nitro methane (nucleophilic donor) and 2-cyclopentenone (electrophilic acceptor).
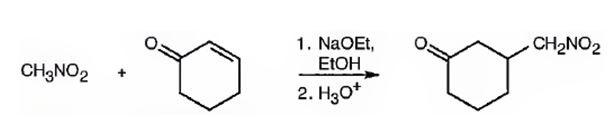
The nucleophilic donor and electrophilic acceptor that react in a Michael reaction to yield the compound shown are
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- Show how the following compound could be prepared by a Suzuki reaction (Bn = benzyl).arrow_forwardWhat products are obtained by hydration of the symmetrical alkyne shown below? Show the mechanism.arrow_forwardGive necessary reagents to make the carbonyl below from an alkyne and also from an akyl halide without altering the number of carbons.arrow_forward
- Predict the products of reaction of pent-1-yne with the following reagents.(a) 1 equivalent of HCl (b) 2 equivalents of HCl (c) excess H2, Ni(d) H2, Pd>BaSO4, quinolinearrow_forwardThe enolate derived from diethyl malonate reacts with a variety of electrophiles (not just alkyl halides) to form new carbon–carbon bonds. With this in mind, draw the products formed when Na+ −CH(CO2Et)2 reacts with each electrophile, followed by treatment with H2O.arrow_forwardFill in the appropriate reagents for the following reaction:arrow_forward
- What is the best set of reagents to use for the synthesis?arrow_forwardPlease propose a mechanism for the following intramolecular Friedel-Crafts Alkylation reaction. Show the mechanism for the formation of the Super Electrophile. Thank you.arrow_forwardPredict the product(s) you would expect from treatment of each compound with (1) dilute,neutral KMnO4 and (2) warm basic KMnO4, then dilute acid. 2-methylhex-3-ynearrow_forward
- Rank the following carboxylic acid derivatives in order of increasing reactivity in nucleophilic substitution reactionsarrow_forwardWhat is the first step in a base catalyzed aldol reaction? A. Protonation of Carbonyl group B. Elimination of Carbonyl with an alkene C. Formation of an Enolate D. Elimination of hydroxide E. Addition of nucleophile to R2C=Oarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


