
(a)
Interpretation: The aldol product that formed by the given compound is to be drawn.
Concept introduction: Aldol reaction is the condensation reaction of the
Answer to Problem 24.1P
The aldol product that formed by the given compound is
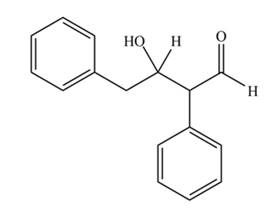
Explanation of Solution
The aldol product that formed by the given compound is shown below.
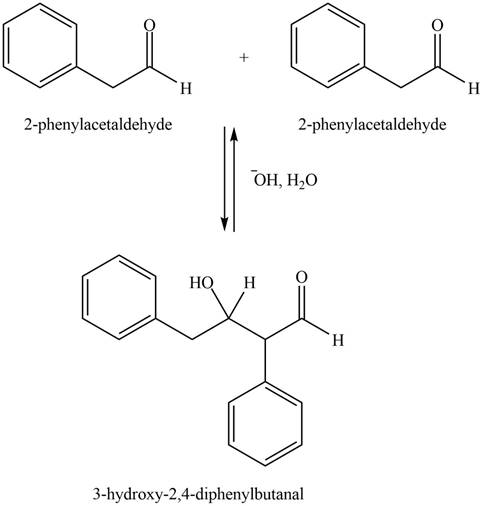
Figure 1
In this aldol reaction, one equivalent of
The aldol product that formed by the given compound is
(b)
Interpretation: The aldol product that formed by the given compound is to be drawn.
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.1P
The aldol product that formed by the given compound is
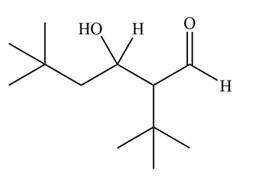
Explanation of Solution
The aldol product that formed by the given compound is shown below.
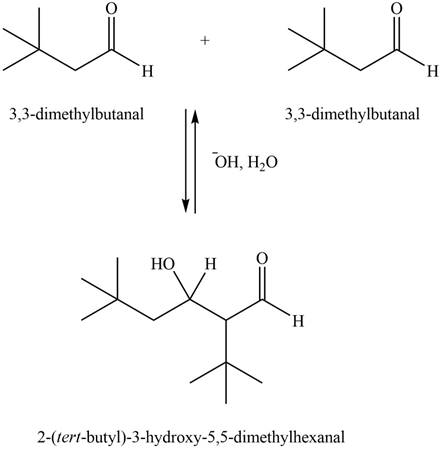
Figure 2
In this aldol reaction, one equivalent of
The aldol product that formed by the given compound is
(c)
Interpretation: The aldol product that formed by the given compound is to be drawn.
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.1P
The aldol product that formed by the given compound is
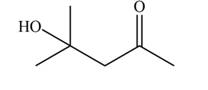
Explanation of Solution
The aldol product that formed by the given compound is shown below.
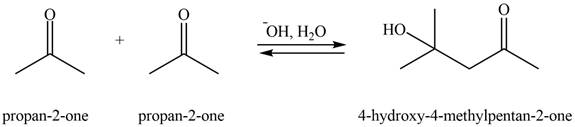
Figure 3
In this aldol reaction, one equivalent of
The aldol product that formed by the given compound is
(d)
Interpretation: The aldol product that formed by the given compound is to be drawn.
Concept introduction: Aldol reaction is the condensation reaction of the organic chemistry. In this reaction an enolate ion or an enol reacts with the carbonyl compound that leads to the formation of
Answer to Problem 24.1P
The aldol product that formed by the given compound is
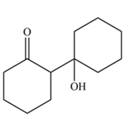
Explanation of Solution
The aldol product that formed by the given compound is shown below.
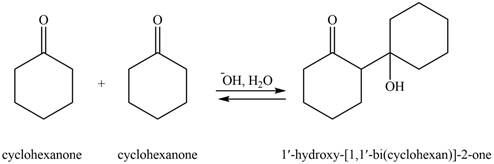
Figure 4
In this aldol reaction, one equivalent of cyclohexanone is treated with strong base that results in the formation of resonance-stabilized enolate ion. The second equivalent of cyclohexanone is then reacts with enolate ion. After that, hydrolysis of the intermediate compound takes place that results the formation of the desired product,
The aldol product that formed by the given compound is
Want to see more full solutions like this?
Chapter 24 Solutions
ORG CHEM LL W/ LL SG&CONPLUS PKG>IC<
- β-Vetivone is isolated from vetiver, a perennial grass that yields a variety ofcompounds used in traditional eastern medicine, pest control, and fragrance. In one synthesis, ketone A is converted to β-vetivone by a two-step process: Michael reaction, followed by intramolecular aldol reaction. (a) What Michael acceptor is needed for the conjugate addition? (b) Draw a stepwise mechanism for the aldol reaction, which forms the six-membered ring.arrow_forwardDevise a synthesis of each compound from the given starting material(s). Albuterol is a bronchodilator and proparacaine is a local anesthetic.arrow_forwardDraw a stepwise mechanism for the following hydrolysis.arrow_forward
- Draw a stepwise mechanism for the sulfonation of an alkyl benzene such as A to form asubstituted benzenesulfonic acid B. Treatment of B with base forms a sodium salt C that canbe used as a synthetic detergent to clean away dirt.arrow_forwardDraw a stepwise mechanism for the attached Friedel–Crafts acylationarrow_forwardDraw a stepwise mechanism for the following reaction:arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





