
Concept explainers
Draw the product of each Robinson annulation from the given starting materials using
a. 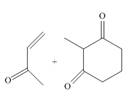 c.
c. 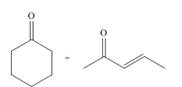
b. 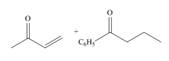 d.
d. 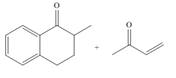
(a)
Interpretation: The product that is formed by the Robinson annulation reaction from the given starting materials by using
Concept introduction: The combination of the Michael reaction and intramolecular aldol reaction in which the formation of a ring takes place is known as Robinson annulation reaction.
In this reaction, the carbonyl compound is treated with a base which leads to the elimination of a proton from the
Answer to Problem 24.45P
The product that is formed by the Robinson annulation reaction from the given starting materials by using
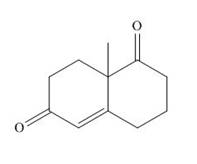
Explanation of Solution
The product that is formed by the Robinson annulation reaction from the given starting materials by using
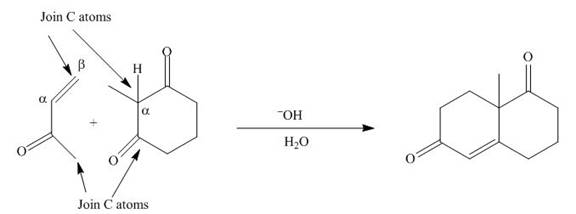
Figure 1
In this reaction, the given dicarbonyl compound is treated with a base,
The product that is formed by the Robinson annulation reaction from the given starting materials by using
(b)
Interpretation: The product that is formed by the Robinson annulation reaction from the given starting materials by using
Concept introduction: The combination of the Michael reaction and intramolecular aldol reaction in which the formation of a ring takes place is known as Robinson annulation reaction.
In this reaction, the carbonyl compound is treated with a base which leads to the elimination of a proton from the
Answer to Problem 24.45P
The product that is formed by the Robinson annulation reaction from the given starting materials by using
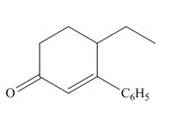
Explanation of Solution
The product that is formed by the Robinson annulation reaction from the given starting materials by using
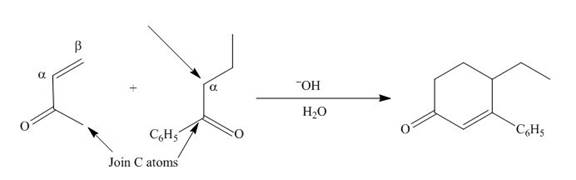
Figure 2
In this reaction, the given carbonyl compound is treated with a base,
The product that is formed by the Robinson annulation reaction from the given starting materials by using
(c)
Interpretation: The product that is formed by the Robinson annulation reaction from the given starting materials by using
Concept introduction: The combination of the Michael reaction and intramolecular aldol reaction in which the formation of a ring takes place is known as Robinson annulation reaction.
In this reaction, the carbonyl compound is treated with a base which leads to the elimination of a proton from the
Answer to Problem 24.45P
The product that is formed by the Robinson annulation reaction from the given starting materials by using
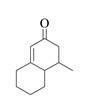
Explanation of Solution
The product that is formed by the Robinson annulation reaction from the given starting materials by using
Figure 3
In this reaction, the given carbonyl compound is treated with a base,
The product that is formed by the Robinson annulation reaction from the given starting materials by using
(d)
Interpretation: The product that is formed by the Robinson annulation reaction from the given starting materials by using
Concept introduction: The combination of the Michael reaction and intramolecular aldol reaction in which the formation of a ring takes place is known as Robinson annulation reaction.
In this reaction, the carbonyl compound is treated with a base which leads to the elimination of a proton from the
Answer to Problem 24.45P
The product that is formed by the Robinson annulation reaction from the given starting materials by using
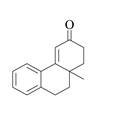
Explanation of Solution
The product that is formed by the Robinson annulation reaction from the given starting materials by using
Figure 4
In this reaction, the given carbonyl compound is treated with a base,
The product that is formed by the Robinson annulation reaction from the given starting materials by using
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry
- Show how to prepare each Gilman reagent from an appropriate alkyl or vinylic halide. Q.Nonanearrow_forwardDraw the product formed by treating each compound with excess CH3I, followed by Ag2O, and then heat.arrow_forwardWhat starting materials are needed to prepare each compound using a Heck reaction?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


