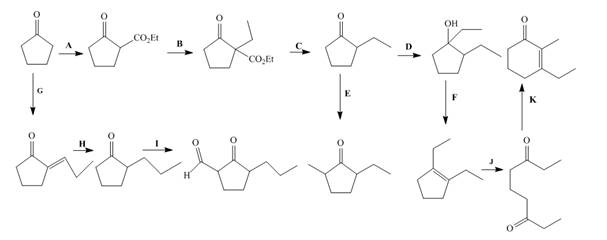
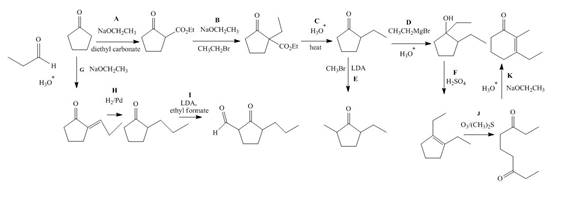
The lettered reagent A is shown as,
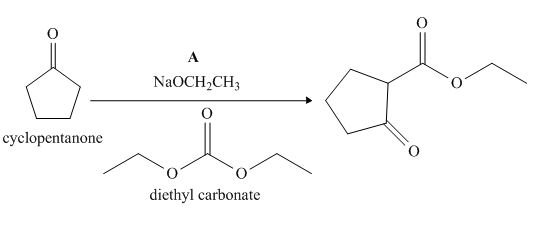
Figure 1
The given compound, cyclopentanone is treated with the base, sodium ethoxide that results in the formation of an enolate ion. Then, the enolate ion reacts with the compound, diethyl carbonate to form the desired compound, ethyl 2−oxocyclopentanecarboxylate. Thus, the reagent A is NaOEt/diethyl carbonate.
The lettered reagent B is shown as,
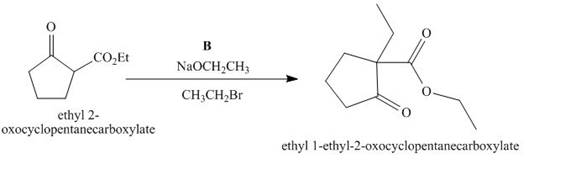
Figure 2
The compound, ethyl 2−oxocyclopentanecarboxylate is again treated with the base, sodium ethoxide that results in the formation of an enolate ion. Then, the enolate ion reacts with the compound, ethyl bromide to form the desired compound, ethyl 1−ethyl−2−oxocyclopentanecarboxylate. Thus, the reagent B is NaOEt/CH3CH2Br.
The lettered reagent C is shown as,
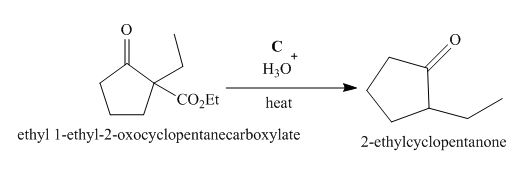
Figure 3
The compound, ethyl 1−ethyl−2−oxocyclopentanecarboxylate undergoes hydrolysis in the presence of hydronium ions followed by the heating of the reaction mixture. The desired product that is obtained by this reaction is 2−ethylcyclopentanone. Thus, the reagent C is H3O+/heat.
The lettered reagent D is shown as,
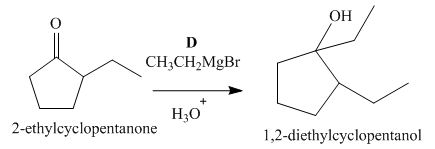
Figure 4
The compound, 2−ethylcyclopentanone is treated with methyl magnesium bromide. Then, the hydrolysis of the reaction mixture in the presence of the hydronium ion takes place. The desired product that is obtained by this reaction is 1,2−diethylcyclopentanol. Thus, the reagent D is CH3CH2MgBr/H3O+.
The lettered reagent E is shown as,
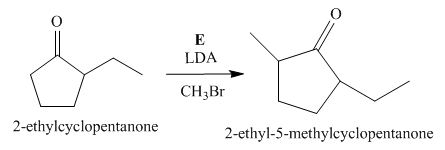
Figure 5
The compound, 2−ethylcyclopentanone is again treated with the base, LDA that abstracts a proton from the compound that leads to the formation of an enolate ion. Then, the enolate ion reacts with methyl bromide to form the desired compound, 2−ethyl−5−methylcyclopentanone. Thus, the reagent E is LDA/CH3Br.
The lettered reagent F is shown as,
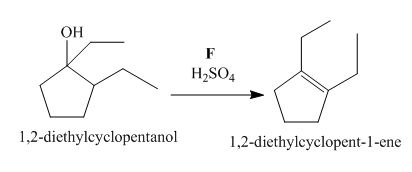
Figure 6
The compound, 1,2−diethylcyclopentanol is treated with the acid, sulfuric acid that results in the dehydration of the compound to form the desired compound, 1,2−diethylcyclopent−1−ene. Thus, the reagent F is H2SO4.
The lettered reagent G is shown as,
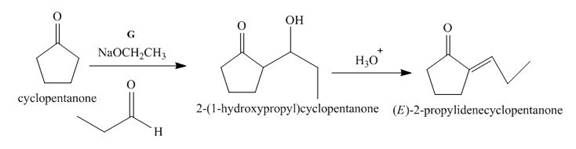
Figure 7
The given compound, cyclopentanone is treated with the base, sodium ethoxide that results in the formation of an enolate ion. Then, the enolate ion reacts with the compound, propanal to form the compound, 2−(1−hydroxypropyl)cyclopentanone followed by the dehydration of this obtained compound. The hydrolysis leads to the formation of the desired compound, (E)−2−propylidenecyclopentanone. Thus, the reagent G is NaOEt/propanol/H3O+.
The lettered reagent H is shown as,
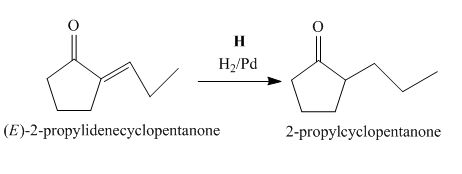
Figure 8
The compound, (E)−2−propylidenecyclopentanone is treated with H2/Pd for the reduction process to form the desired compound, 2−propylcyclopentanone. Thus, the reagent H is H2/Pd.
The lettered reagent I is shown as,
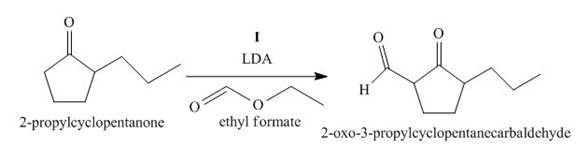
Figure 9
The compound, 2−propylcyclopentanone is treated with the base, LDA that abstracts a proton from the compound that leads to the formation of an enolate ion. Then, the enolate ion reacts with ethyl formate to form the desired compound, 2−oxo−3−propylcyclopentanecarbaldehyde. Thus, the reagent I is LDA/ethyl formate.
The lettered reagent J is shown as,
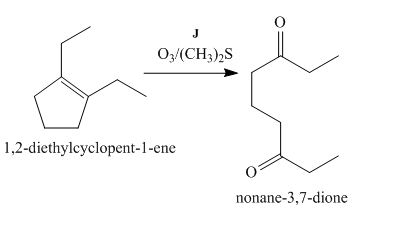
Figure 10
The compound, 1,2−diethylcyclopent−1−ene is treated with ozone and (CH3)2S that abstracts a proton from the compound that leads to the formation of an enolate ion. Then, the enolate ion reacts with ethyl formate to form the desired compound, nonane−3,7−dione. Thus, the reagent J is O3/(CH3)2S.
The lettered reagent K is shown as,
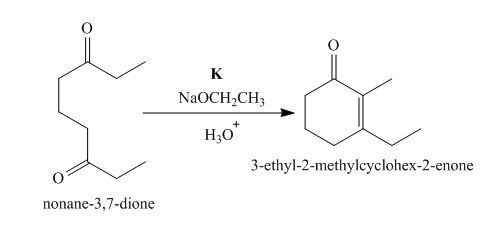
Figure 11
The intramolecular aldol reaction takes place in the compound, nonane−3,7−dione in the presence of sodium ethoxide. Then, the dehydration of the intermediate takes place in the presence of hydronium ion to form the desired product, 3−ethyl−2−methylcyclohex−2−enone Thus, the reagent K is NaOEt/H3O+.
The complete filled reagents that are needed for the given reactions are shown as,
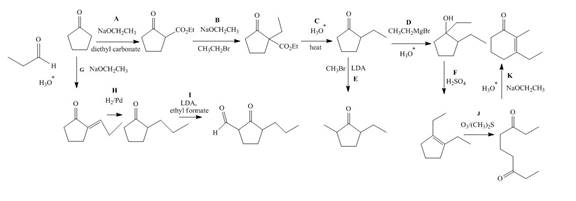
Figure 12