
Concept explainers
Answer the following questions about
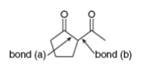
a. What starting materials are needed to form
b. What starting materials are needed to form
c. What product is f ormed when
d. Draw the Robinson annulation product(s) formed by reaction of
e. Draw the structure of the most stable enol tautomer(s).
(a)
Interpretation: The starting materials needed to form
Concept introduction: In crossed Claisen condensation reaction, a base abstracts an acidic proton from an
Answer to Problem 24.65P
The starting materials needed to form
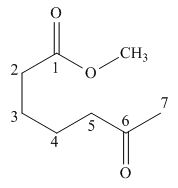
Figure 1
Explanation of Solution
The given compound is,
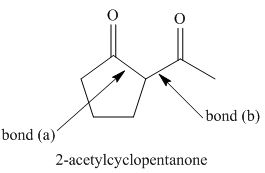
Figure 2
The starting materials needed to form

Figure 1
The base abstracts the proton from
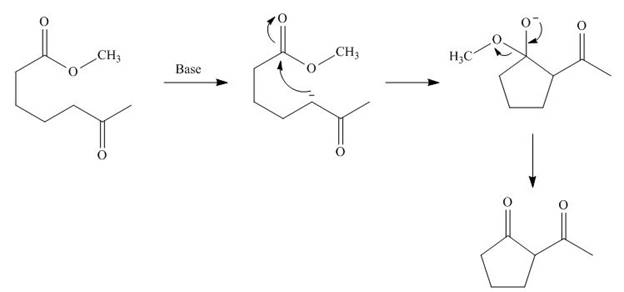
Figure 3
The starting materials needed to form
(b)
Interpretation: The starting materials needed to form
Concept introduction: In crossed Claisen condensation reaction, a base abstracts an acidic proton from an
Answer to Problem 24.65P
The starting materials needed to form
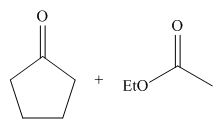
Figure 4
Explanation of Solution
The given compound is,

Figure 2
The starting materials needed to form

Figure 4
The base abstracts the proton from
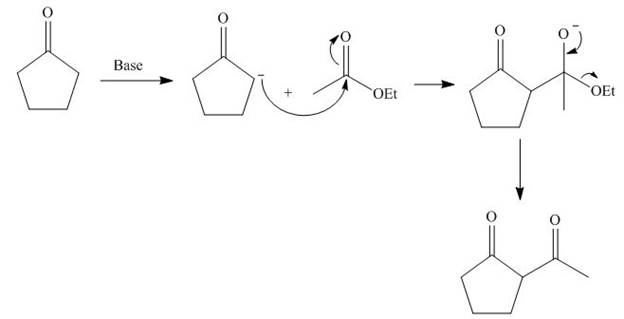
Figure 5
The starting materials needed to form
(c)
Interpretation: The product formed on treatment of
Concept introduction: Alkylation of carbonyl compounds takes place in the presence of a strong base. The use of appropriate base, solvent and temperature can yield one major product regioselectively. The first step is abstraction of proton and second step is attack of electrophile to form alkylated product.
Answer to Problem 24.65P
The product formed on treatment of
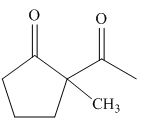
Figure 6
Explanation of Solution
The product formed on treatment of
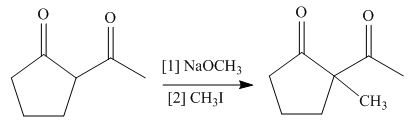
Figure 7
The base
The product formed on treatment of
(d)
Interpretation: The products formed on Robinson annulations of
Concept introduction: Robinson annulation is a combination of Michael addition and intramolecular aldol condensation reactions. It takes place between
Answer to Problem 24.65P
The products formed on Robinson annulations of
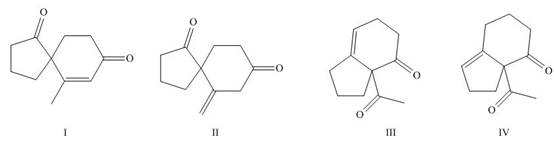
Figure 8
Explanation of Solution
The first step is the Michael addition between
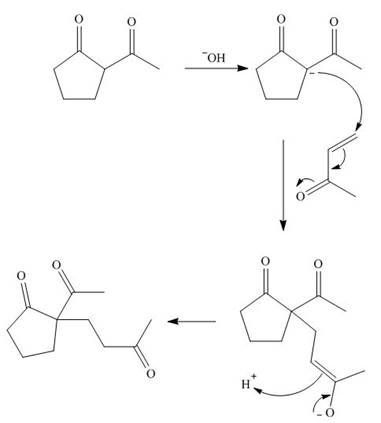
Figure 9
The base abstracts the proton between the two carbonyl groups to form enolate. The enolate formed gives the conjugate addition on methyl vinyl ketone followed by protonation resulting in
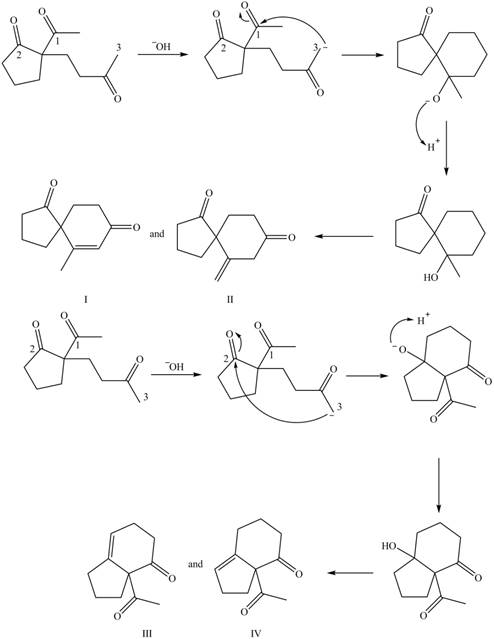
Figure 10
The base abstracts the acidic proton from the
The products formed on Robinson annulations of
(e)
Interpretation: The structure of the most stable enol form tautomer is to be drawn.
Concept introduction: Keto and enol form of a compound exists in the chemical equilibrium with each other. Enol form refers to the compound in which carbonyl group exist as hydroxyl group adjacent to carbon-carbon double bond.
Answer to Problem 24.65P
The structure of the most stable enol form tautomer is,
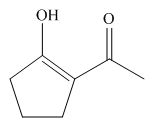
Figure 11
Explanation of Solution
The enol form tautomer of
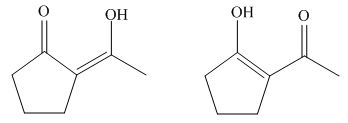
Figure 12
The stability of enols depends on the stability of alkenes. The alkene with endo double bond is more stable in case of five membered ring.
Thus, the most stable enol form tautomer is,

Figure 11
The structure of the most stable enol form tautomer is shown in Figure 11.
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry
- How Wittig reagents are synthesized by a two-step procedure ?arrow_forward1. a. 4-methoxybenzoic acid is less or more polar than 4-methoxyacetophenone? explain why (WITHOUT DRAWINGS) b. 3′-chloro-4′-methoxyacetophenone is less or more polar than 4-methoxyacetophenone? explain why (WITHOUT DRAWINGS)arrow_forwardDraw the products formed when A or B is treated with each reagent. In some cases, no reaction occurs.a. NaBH4, CH3OHb. [1] LiAlH4; [2] H2Oc. [1] CH3MgBr (excess); [2] H2Od. [1] C6H5Li (excess); [2] H2Oe. Na2Cr2O7, H2SO4, H2Oarrow_forward
- Give the structure corresponding to each name: (a) sec-butyl ethyl ketone; (b) methyl vinyl ketone; (c) p-ethylacetophenone; (d) 3-benzoyl-2-benzylcyclopentanone; (e) 6,6-dimethylcyclohex-2-enone; (f) 3-ethylhex-5-enal.arrow_forwardWhat starting materials are needed to prepare each compound using a Michael reaction?arrow_forwardDraw a stepwise mechanism for the sulfonation of an alkyl benzene such as A to form a substituted benzenesulfonic acid B. Treatment of B with base forms a sodium salt C that can be used as a synthetic detergent to clean away dirt (see Problem 3.22).arrow_forward
- 13.6 What reagents to make ether with Williamson synthesis?arrow_forwardWhat compound results when 2-pentanol is treated with HCl and ZnCl2? A. CH3CH2CH2CH2CH2CL B. CH3CH(Cl)CH2CH2CH3 C. CH3CH2CH2CH2CH2ZnCl D. CH3CH2CH2Ch2CO2Clarrow_forwardWhats the reason for the girgnard reagent attack that carbon of starting point in this step? a. Bc it gives us desired product b. Due to the sterics c. Bc it is more electrophilic than the other carbon of the starting pointarrow_forward
- Draw the structure corresponding to each name.a. 5-methylheptanoyl chloride b. isopropyl propanoatec. acetic formic anhydride d. N-isobutyl-N-methylbutanamide e. sec-butyl 2-methylhexanoate f. N-ethylhexanamidearrow_forwardAnswer the following question about curcumin, a yellow pigmentisolated from turmeric, a tropical perennial in the ginger family and aprincipal ingredient in curry powder. Most enols, compounds that contain a hydroxy group bonded to a C=C, are unstable and tautomerize to carbonyl groups. Draw the keto form of the enol of curcumin, and explain why the enol is more stable than many other enols.arrow_forwardwhat product is formed after recrystallizing a mixture of 4-phenylcyclohexanone and 4-phenylcyclohexanol?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
