
Concept explainers
(a)
To determine: The line–angle structural formula for the activated form of lauric acid.
Answer to Problem 24.71UTC
Solution: The line–angle structural formula for the activated form of lauric acid is:
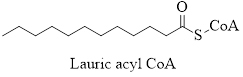
Explanation of Solution
Lauric acid is a saturated fatty acid. It can be activated by Coenzyme A catalysed by Acyl CoA synthetase.
Thus, the activated form of Lauric acid is:
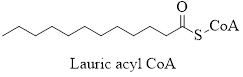
(b)
To determine: The α- and β-carbon atoms in the acyl molecule of lauric acid.
Answer to Problem 24.71UTC
Solution: The α- and β-carbon atoms in the acyl molecule of lauric acid is:
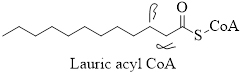
Explanation of Solution
The carbon attached to the carbonyl carbon is considered as α-carbon and the carbon next to α-carbon is called as β-carbon.
Thus the α- and β-carbon atoms in the acyl molecule of lauric acid can be represented as
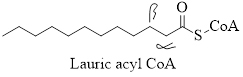
(c)
To determine: The number of cycles of β- oxidation needed for complete β-oxidation of lauric acid.
Answer to Problem 24.71UTC
Solution: The number of cycles of β- oxidation needed for complete β-oxidation of lauric acid is five ().
Explanation of Solution
The acetyl group of the acetyl CoA is formed by two carbons and in the last round two acetyl CoA is produced. Accordingly the number of cycles of β-oxidation has been calculated.
No. of cycles of β-oxidation are needed for the complete oxidation of fatty acid = (n/2)-1 where n = No. of carbon atoms present in fatty acid.
As here no. of carbon atoms in the given fatty acid = 12
So, by putting n = 12
We get, No. of cycles of β-oxidation are needed for the complete oxidation of fatty acid = (12/2) -1 = 5
Thus, five () cycles of β-oxidation are needed for the complete oxidation of lauric acid.
(d)
To determine: The number of acetyl CoA units produced from the complete β-oxidation of lauric acid.
Answer to Problem 24.71UTC
Solution: The number of acetyl CoA units produced from the complete β-oxidation of lauric acid is six ().
Explanation of Solution
The acetyl group of the acetyl CoA is formed by two carbons. Accordingly the number of acetyl CoA produced has been calculated.
No. of acetyl CoA are produced from the complete oxidation of fatty acid = (n/2)
where n = No. of carbon atoms present in fatty acid.
As here no. of carbon atoms in the given fatty acid = 12
So, by putting n=12
We get, No. of acetyl CoA are produced from the complete oxidation of fatty acid = (12/2) = 6
Thus, from complete oxidation of lauric acid six(6) acetyl CoA are produced.
(e)
To determine: The total ATP yield from the complete β-oxidation of lauric acid.
Answer to Problem 24.71UTC
Solution: The total ATP yield from the complete β-oxidation of lauric acid is .
Explanation of Solution
From the complete β-oxidation of lauric acid, total six(6) acetyl CoA, 5 NADH and 6 FADH2 has been produced. Each Acetyl CoA yields 10 ATP, each NADH yields 2.5 ATP and each FADH2 yields 1.5 ATP. Accordingly, ATP yield has been calculated.
Lauric Acid is a C12 fatty acid. And for the activation of lauric acid 2 ATP is required.
| Activation | -2 ATP | |
| Acetyl CoA | 6x10ATP/Acetyl CoA | 60ATP |
| NADH.H | 5x2.5 ATP/NADH | 12.5 ATP |
| FADH2 | 5x1.5 ATP/ FADH2 | 7.5 ATP |
| Total | 78 ATP |
Thus, 78 ATP yields from the complete oxidation of lauric acid.
Therefore, the line-angle structural formula for the activated form, α- and β-carbon atoms in the acyl molecule, number of cycles of β- oxidation, number of acetyl CoA units produced and the total ATP yield from the complete β- oxidation of lauric acid has been discussed.
Want to see more full solutions like this?
Chapter 24 Solutions
General, Organic, and Biological Chemistry: Structures of Life (5th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





