
Interpretation:
When the starting
Concept Introduction:
Heck reaction:
A palladium-catalyzed reaction of the carbon group of a haloalkene substituted for a hydrogen on double bonded carbon of an alkene is said to be Heck reaction.
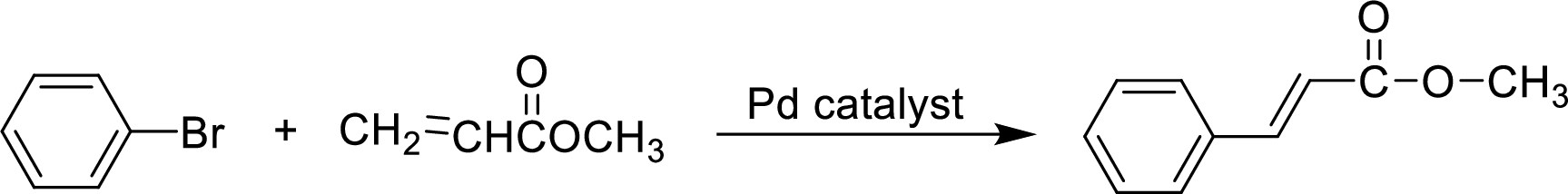
Explanation of Solution
The given reaction is
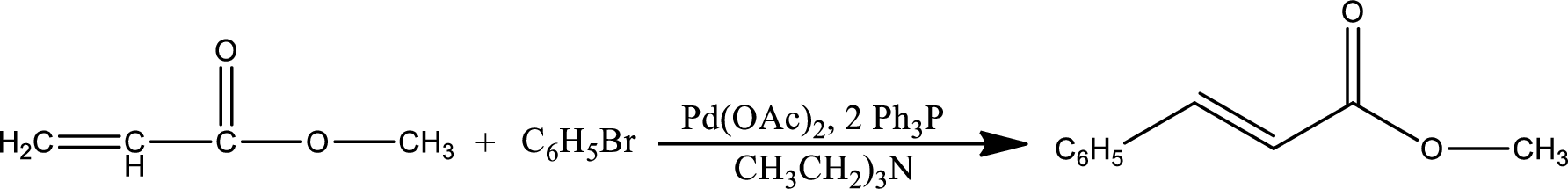
Mechanism:
Step-1:
Formation of Heck catalyst:
Heck catalyst s formed by the reduction of palladium from
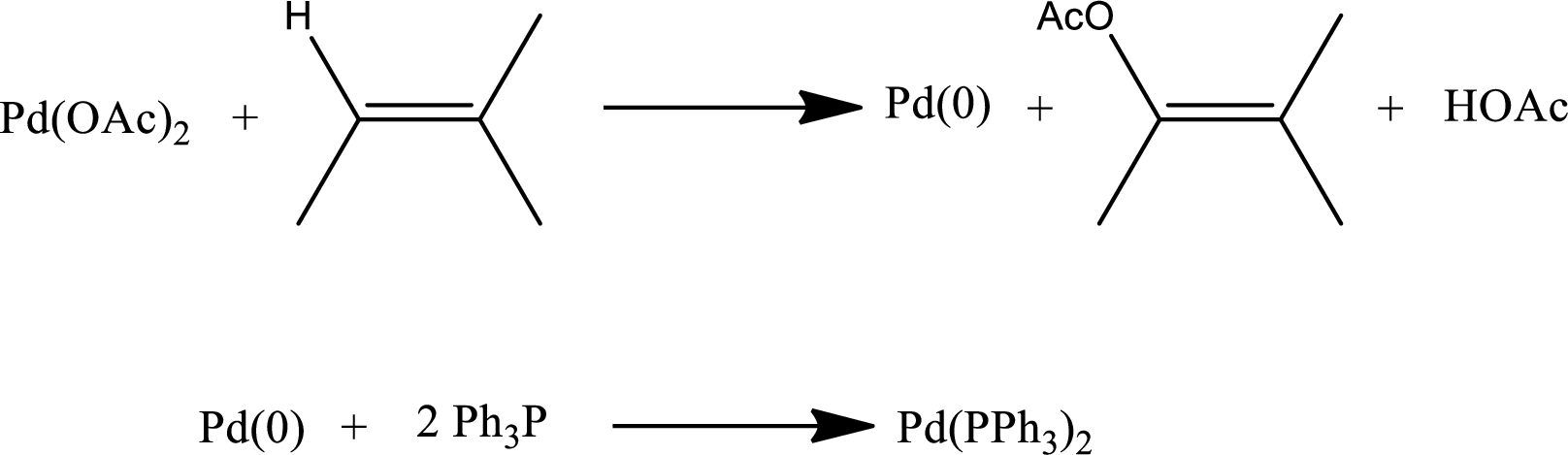
Step-2:
Catalytic cycle for the formation of product:
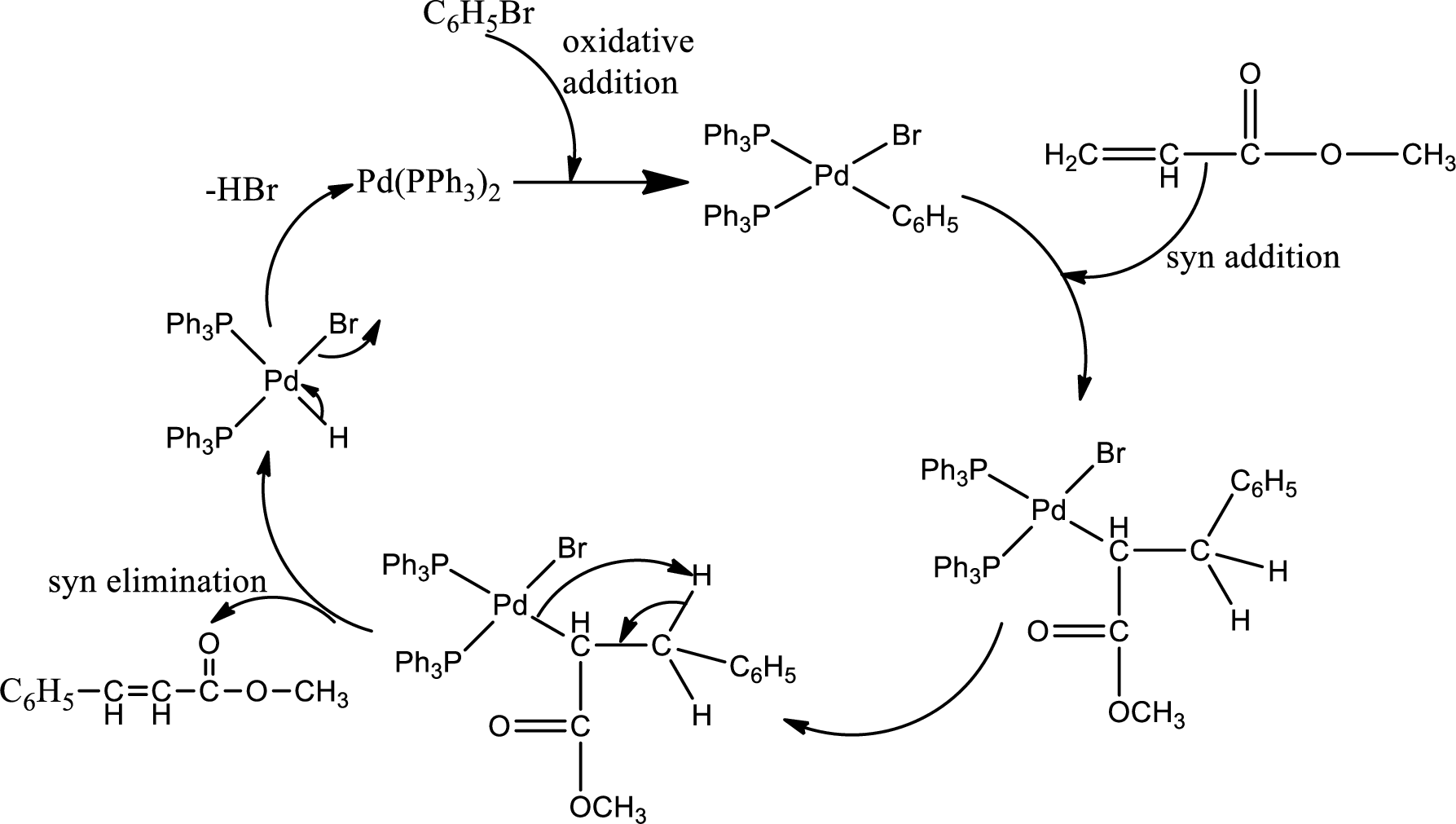
The addition of alkene to the catalyst is syn addition and elimination of product is also syn elimination. The inversion of configuration occurs in the addition and forms E-product.
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry
- Order each of the following sets of compounds with respect to SN1 reactivity.arrow_forwardWhen the compound shown below undergoes acid-catalyzed dehydration, a ring expansionoccurs to give the fused-ring product. What type of intermediate is formed in this reaction?Explain the ring expansion in terms of the reaction mechanismarrow_forwardConsider the following statement in reference to SN1, SN2, E1, and E2 reactions of haloalkanes. To which mechanism(s), if any, does the statement apply? Substitution at a stereocenter gives predominantly a racemic productarrow_forward
- A student prepares a reaction in which hexane is converted to 1,3-hexadiene. In this reaction, what process does hexane undergo? Please explain in detail.arrow_forwardConversion of an alkene to a halohydrin and internal displacement of a halide ion by an alkoxide ion are both stereoselective. Use this information to demonstrate that the configuration of the alkene is preserved in the epoxide. As an illustration, show that reaction of cis-2-butene by this two-step sequence gives cis-2,3- dimethyloxirane (cis-2-butene oxide).arrow_forwardProvide the mechanism for the Friedel-Crafts acylation reaction of compound Q and 2-(1- phenylethyl)-1,3-dioxolane that leads to the formation of intermediate R.arrow_forward
- Dialkyl peroxides are a family of compounds which can be explosive when heated. These explosions begin with the following bond fragmentation (which is then followed by other reaction steps). Which process best describes this fragmentation? E2 Elimination Heterolytic dissociation Homolytic dissociation Racemizationarrow_forwardShow the process of synthesizing 2-methyl2-cyclohexenone from 2-methyl cyclohexanone. Write down the stages of the monocyclic reaction process in detail.arrow_forwardPredict whether the following reactions will proceed via SN1, SN2, E1, E2, or if the substrate will not react under the given conditions. Draw all product(s) with the correct stereochemistry for your proposed mechanism. Write a sentence or two discussing the nature of the substrate, nucleophile, leaving group, solvent, etc. that helped you arrive at your answer.arrow_forward
- Give the product/s for the following reaction and indicate what mechanism is involved in the formation of such product/s as SN1, SN2, E1, E2 BrCH2CH2CH2Br + Mg (ether)arrow_forwardProvide a drawing that explains the formation of the major product of this reaction. - Use resonance structures to explain the regiochemistry of this reaction, and provide a detailed mechanism of this reaction.arrow_forwardIn the chemical reaction between 2-chloro-2-methyl-propane and ethanolic silver nitrate solution, a precipitate is definitely formed. Using the skeletal structures of the molecules, write out the skeletal structure of each reaction (don't include the mechanism). Identify what the nucleophile is in each type of reaction, especially within this SN1 reaction. Explain the reactivity of each electrophile/substrate in terms of whether they are tertiary, secondary, primary, etc. for each reaction. Include the overall chemical reaction.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
