
Concept explainers
Interpretation:
The value of
Concept introduction:
For enzymatic reaction, the common equation is given below,
Here,
E is the enzyme.
S is the substrate.
ES is the enzyme substrate complex.
P is the product.
The general equation for Michaelis-Menten is dervied from above equation,
Here,
Take the Reciprocal of both the sides in equation (1),
This equation is called as Lineweaver-Burk equation. By using this equation the value of
Explanation of Solution
The
Given:
The substare concentration and
By using the equation,
Calculate the value of
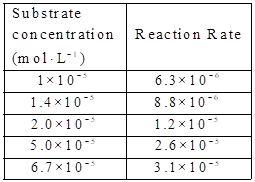
The graph between
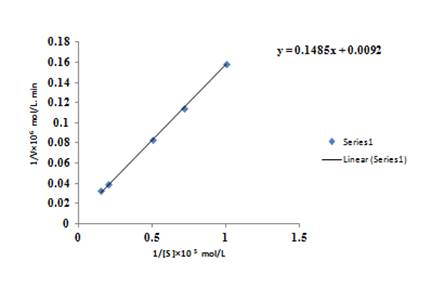
The equation for the straight line obtained from the graph is
Rearrange the equation, in form of
The value of intercept comes from the graph is
Rearrange for
So, the value of
The value of
Want to see more full solutions like this?
Chapter 24 Solutions
Chemistry & Chemical Reactivity
- Fats belong to the class of organic compounds represented by the general formula, RCOOR', where R and R' represent hydrocarbon groups; therefore, fats are: a. ethers. b. soaps. c. esters. d. lipases.arrow_forwardThe following is a block diagram for a glycerophospholipid where the building blocks are labeled with letters and the linkages between building blocks are labeled with numbers. a. Which building blocks are fatty acid residues? b. Which building blocks are alcohol residues? c. Which linkages are ester linkages? d. Which linkages involve a phosphate residue?arrow_forwardComplete the following statements: a. Oxidation of a secondary alcohol produces ___________. b. Oxidation of a primary alcohol produces an aldehyde that can be further oxidized to a ________. c. Hydrogenation of a ketone produces __________. d. Hydrogenation of an aldehyde produces __________. e. Hydrolysis of an acetal produces _________. f. Hydrolysis of a ketal produces _________.arrow_forward
- Predict the reactions of lipids under basic hydrolysis and with standard organicreagents. Show the reactions of the ester and olefinic groups of glycerides and thecarboxyl groups of fatty acids.arrow_forwardA simple triacylglycerol is one that: a) Upon hydrolysis produces glycerol and three different fatty acids. b) Upon hydrolysis produces glycerol and at least two different fatty acids. c) Upon saponification gives glycerol and three fatty acid salts. d) Upon saponification gives three esters. e) Upon hydrolysis produces glycerol and three mol of a fatty acid.arrow_forwardAncient peoples used salicin to reduce fevers. Write an equation for the acid-catalyzed hydrolysis of the glycosidic bond of salicin. Compare the aromatic product with the structure of acetylsalicylic acid (aspirin). Use this information to develop a hypothesis explaining why ancient peoples used salicin to reduce fevers.arrow_forward
- Draw a phosphoglyceride formed by the reaction of glycerol, 1 molecule of myristic acid, 1 molecule of linoleic acid, 1 phosphate and 1 molecule of ethanolamine. The drawing should be in the form found at physiological pH. Use a line drawing for the fatty acids. Becarful with the geometry of the unsaturated fatty acid. Make sure you attach the ethanolamine via the correct atom.arrow_forwardGive the structural equation for the formation of the ester methyl butanoate a condensation reaction. Include necessary conditions. Identify the small molecule byproduct and show where the atoms in that byproduct came from. Indicate the new bond in the ester product. Give possible reactants to form the following condensation products and side products heat H + +H-CI Harrow_forwardGive the functions of the following ingredients, then name a branded/commercial skin or hair care product where the said material is used. Tetrasodium EDTA Cholesterol Silicone Oil 200/50 cst BETAINE Carbopol Ultrez D & C Red No. 17 ( CI No. 26100) Bisabolol (Alpha Bisabolol) Ceresin Wax Disodium Lauryl Sulfosuccinate Hydroxypropyl Methylcellulosearrow_forward
- Write the product (s) formed in the following reactions.arrow_forwardTrue or false 4-bromoaniline could also be named o-bromoaniline. A typical unsaturated fatty acid has a trans double bond in its structure. Trytophan is a hydrophillic, non-polar amino acid. Glucose is a substrate. The substrate for the enzyme succinate dehydrogenase is succinate. If hydrogen bonding between amino acids in the same polypeptide give a coiled shape to the protein we have an example of primary protein structure. A salt bridge would be the type of interaction between two leucines in a tertiary protein structure. Isomerases catalyze the bonding of molecules using ATP energy.arrow_forwardWhich fatty acid is likely to occur commonly in natural sources?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,




