
(a)
Interpretation: The products formed by the reaction of given compound with
Concept introduction:
Answer to Problem 25.24P
The products formed by the reaction of given compound with
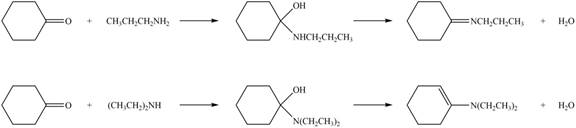
Explanation of Solution
Amines on reaction with acid chlorides and anhydride form corresponding amide whereas on reaction with ketones and aldehydes they form imine or enamine. Primary and secondary amines on reaction with ketone and aldehyde form imine and enamine, respectively.
The given compound is ketone as the carbonyl carbon is bonded to two alkyl groups. The products formed by the reaction of primary amine
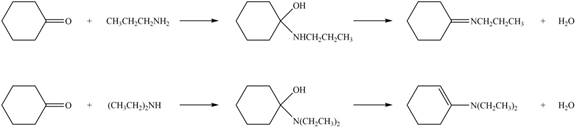
Figure 1
The products formed by the reaction of given compound with
(b)
Interpretation: The products formed by the reaction of given compound with
Concept introduction: Amines on reaction with acid chlorides and anhydride form amide whereas on reaction with ketones and aldehydes they form imine or enamine. Primary and secondary amines on reaction with ketone and aldehyde form imine and enamine, respectively.
Answer to Problem 25.24P
The products formed by the reaction of given compound with
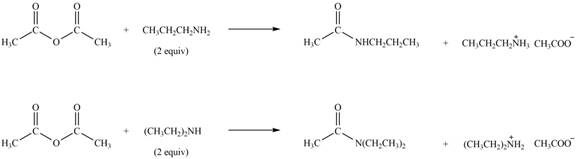
Explanation of Solution
Amines on reaction with acid chlorides and anhydride form corresponding amide whereas on reaction with ketones and aldehydes they form imine or enamine. Primary and secondary amines on reaction with ketone and aldehyde form imine and enamine, respectively.
The given compound is anhydride as two carbonyl carbon atoms are bonded to each other through oxygen atom. The products formed by the reaction of primary amine
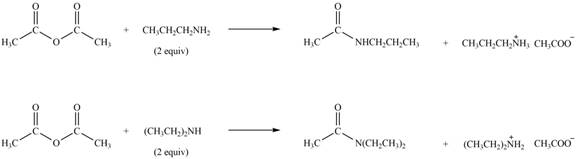
Figure 2
(c)
Interpretation: The products formed by the reaction of given compound with
Concept introduction: Amines on reaction with acid chlorides and anhydride form amide whereas on reaction with ketones and aldehydes they form imine or enamine. Primary and secondary amines on reaction with ketone and aldehyde form imine and enamine, respectively.
Answer to Problem 25.24P
The products formed by the reaction of given compound with
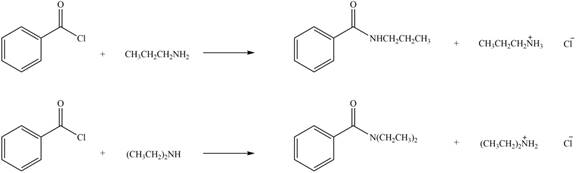
Explanation of Solution
Amines on reaction with acid chlorides and anhydride form corresponding amide whereas on reaction with ketones and aldehydes they form imine or enamine. Primary and secondary amines on reaction with ketone and aldehyde form imine and enamine, respectively.
The given compound is acid chloride as the carbonyl carbon is bonded to alkyl groups and chlorine atom. The products formed by the reaction of primary amine
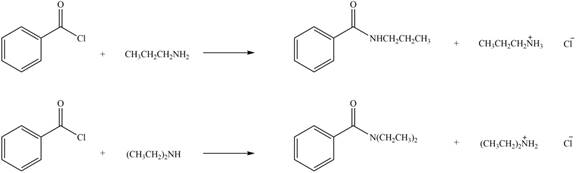
Figure 3
The products formed by the reaction of given compound with
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry
- Fenfluramine and phentermine are two components of fen–phen, an appetite suppressant withdrawn from the market in 1997 after it was shown to damage the heart valves in some patients. What products are formed when fenfluramine and phentermine are each treated with acetic acid (CH3CO2H)?arrow_forwardSafrole, which is isolated from sassafras (Problem 21.33), can be converted to the illegal stimulant MDMA (3,4- methylenedioxymethamphetamine, “Ecstasy”) by a variety of methods. (a) Devise a synthesis that begins with safrole and uses a nucleophilic substitution reaction to introduce the amine. (b) Devise a synthesis that begins with safrole and uses reductive amination to introduce the amine.arrow_forwardReactions of aldehydes and ketones with amines and amine derivatives a. Draw reaction with a primary amine forms an imine. Hydrazine and hydroxylamine can also be used; they form a hydrazone and an oxime, respectively. b. Draw reaction with a secondary amine forms an enamine.arrow_forward
- Safrole, which is isolated from sassafras, can be converted to the illegal stimulant MDMA (3,4- methylenedioxymethamphetamine, “Ecstasy”) by a variety of methods. (a) Devise a synthesis that begins with safrole and uses a nucleophilic substitution reaction to introduce the amine. (b) Devise a synthesis that begins with safrole and uses reductive amination to introduce the amine.arrow_forwardDraw the products formed when each amine is treated with [1] CH3I (excess); [2] Ag2O; [3] Δ. Indicate the major product when a mixture results.arrow_forwardWhat nitro compound, nitrile, and amide are reduced to each compound?arrow_forward
- draw the following d) butylbutanoatee) 3-chloropentanoic acid f) 2,2-dibromo-butanalg) 2-octanolh) 1-propynei) butylethylmethylamine j) butylpropyletherarrow_forwardMany drugs are sold as their hydrochloride salts (R2NH2+ Cl−), formed by reaction of an amine (R2NH) with HCl. a.Draw the product (a hydrochloride salt) formed by reaction of acebutolol with HCl. Acebutolol is a β blocker used to treat high blood pressure. b. Discuss the solubility of acebutolol and its hydrochloride salt in water. c.Offer a reason as to why the drug is marketed as a hydrochloride salt rather than a neutral amine.arrow_forwardWhy is the bond between N and H in amines a polar bond? Hydrogen is more electronegative. B) Nitrogen is more electropositive. C) Nitrogen is more electronegative. D) Hydrogen is electrophilic. E) Nitrogen is electrophilicarrow_forward
- Many drugs are sold as their hydrochloride salts (R2NH2 + Cl−), formed by reaction of an amine (R2NH) with HCl . Question: Draw the product (a hydrochloride salt) formed by reaction ofacebutolol with HCl. Acebutolol is a β blocker used to treat high bloodpressure.arrow_forwardQ1. How are the alkaloids classified? Q2. Give any four biological sources of quinine? Give the isolation and extraction of quinine. Q3. Give the synthesis of capsaicin from vanillin.arrow_forwardShow how to convert amines to other functional groups, and devise multistepsyntheses using amines as starting materials and intermediatesarrow_forward

 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning



