
Concept explainers
(a)
Interpretation: To determine the functional group change that occurs in step 1 of a turn of the
Concept introduction: The
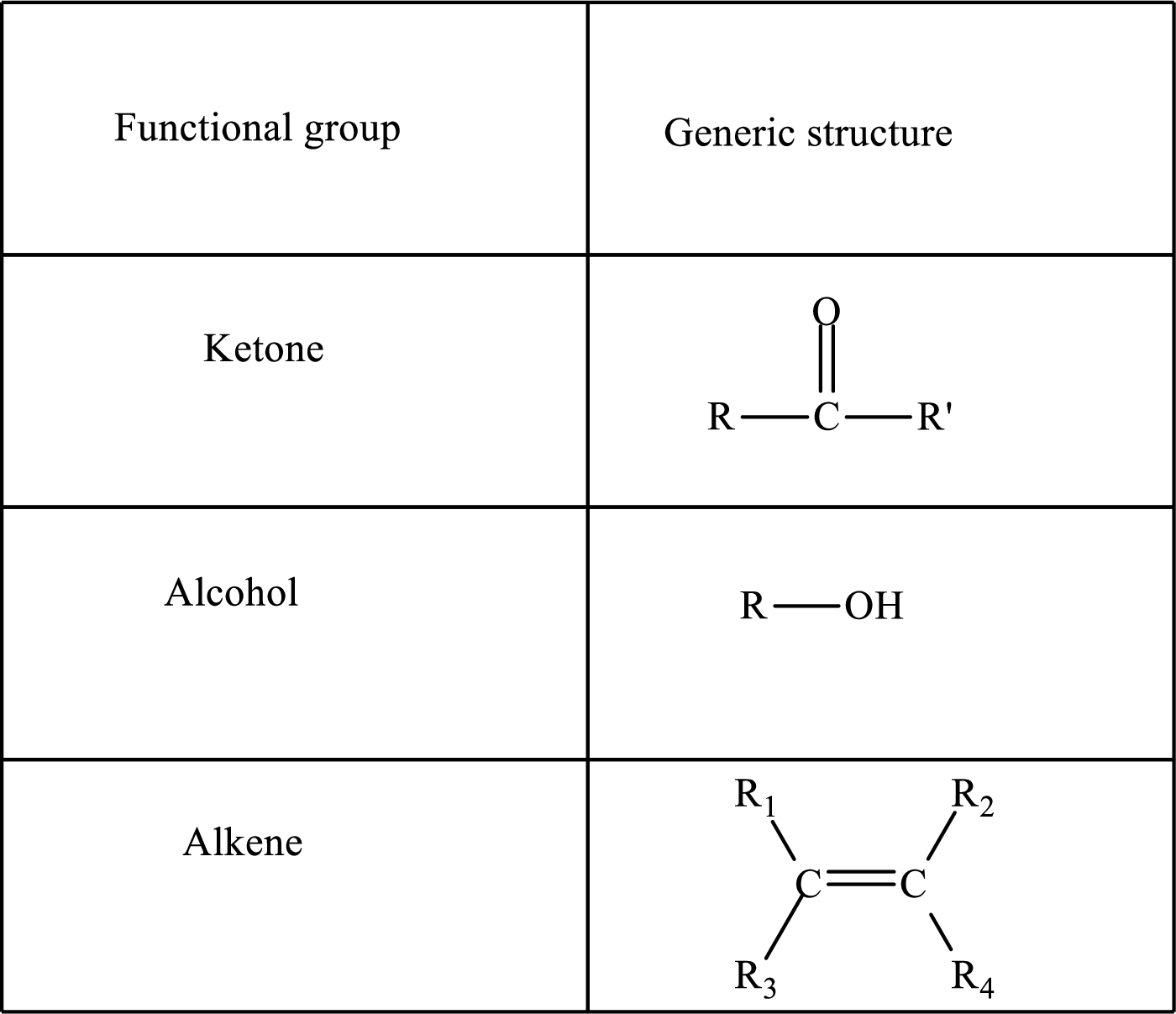
Here, R and
Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms.
(a)
Answer to Problem 25.29EP
In step 1 of a turn of the
Explanation of Solution
The reaction in step 1 of a turn of the
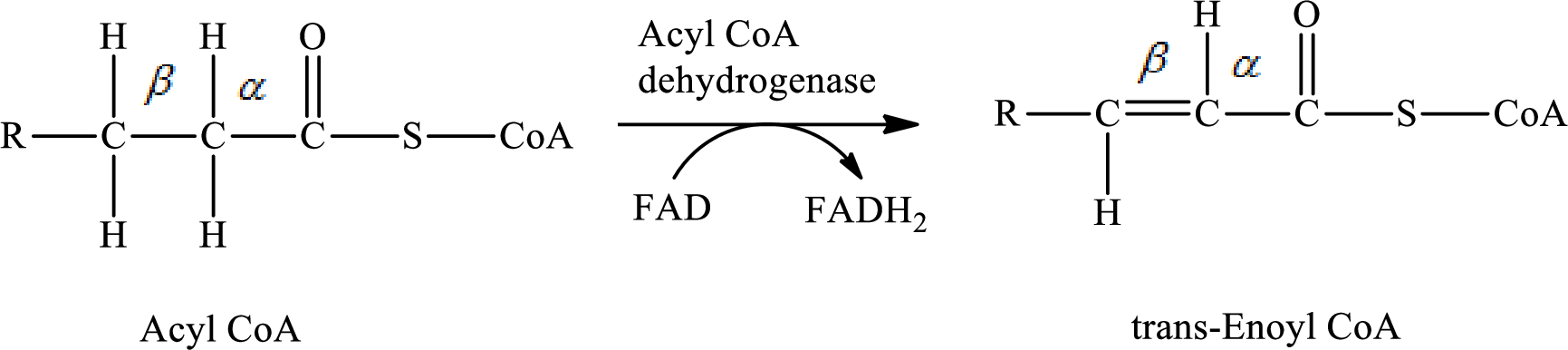
Therefore,
Carbon atoms are bonded with other carbon atoms by a single covalent bond in
(b)
Interpretation: To determine the functional group change that occurs in step 2 of a turn of the
Concept introduction: The
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
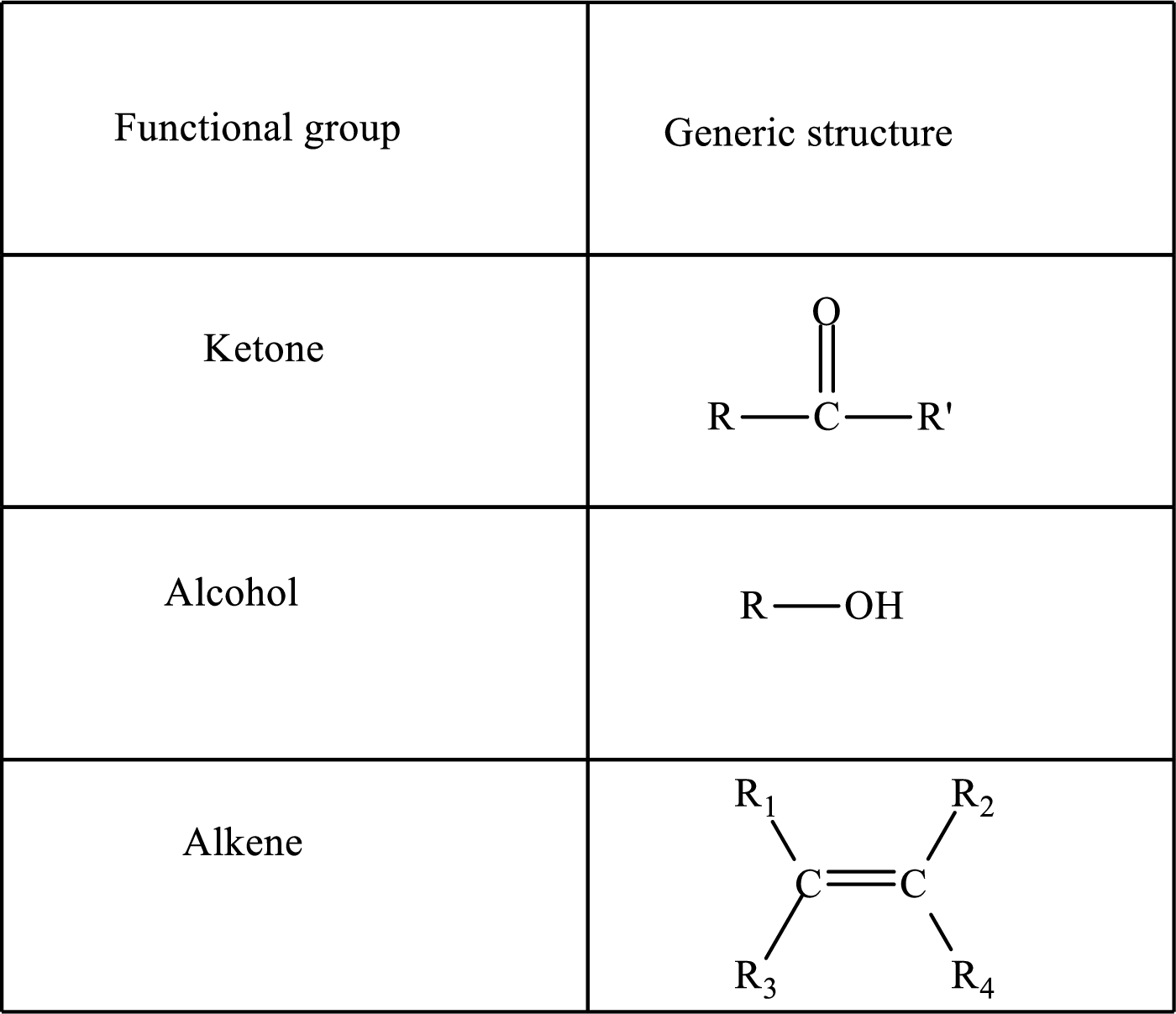
Here, R and
Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. In secondary alcohol, the carbon atom of the hydroxyl group
(b)
Answer to Problem 25.29EP
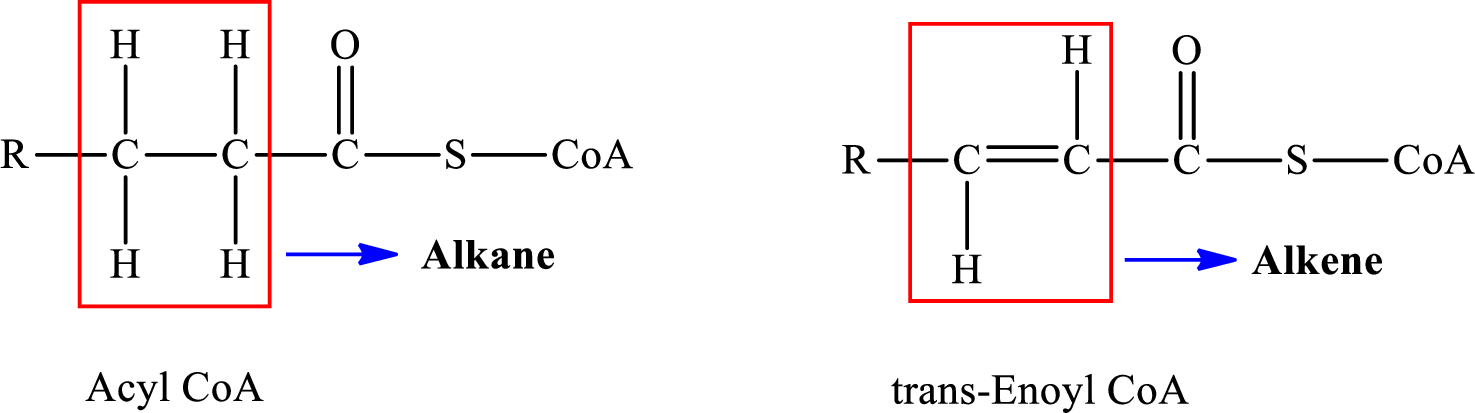
In step 2 of a turn of the
Explanation of Solution
The reaction in step 2 of a turn of the
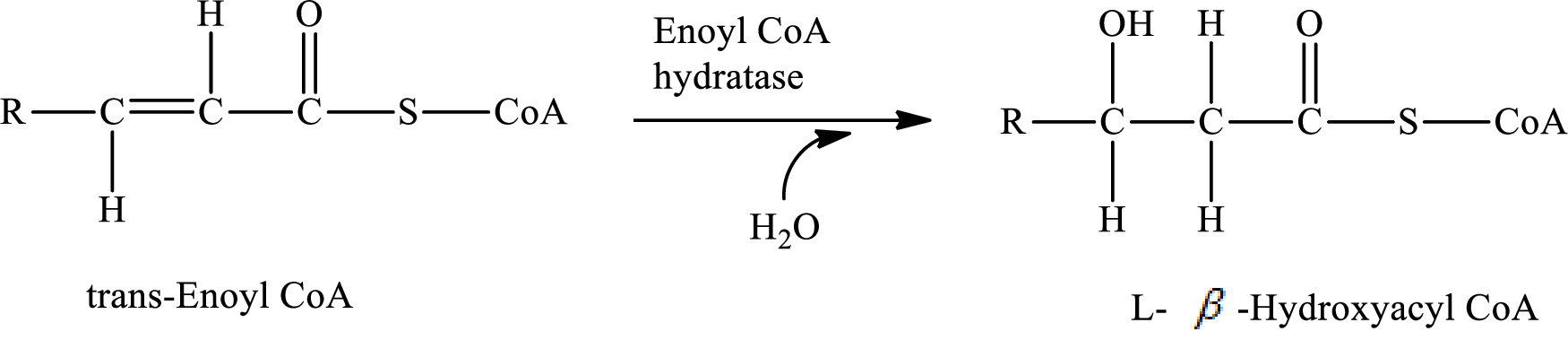
Therefore,
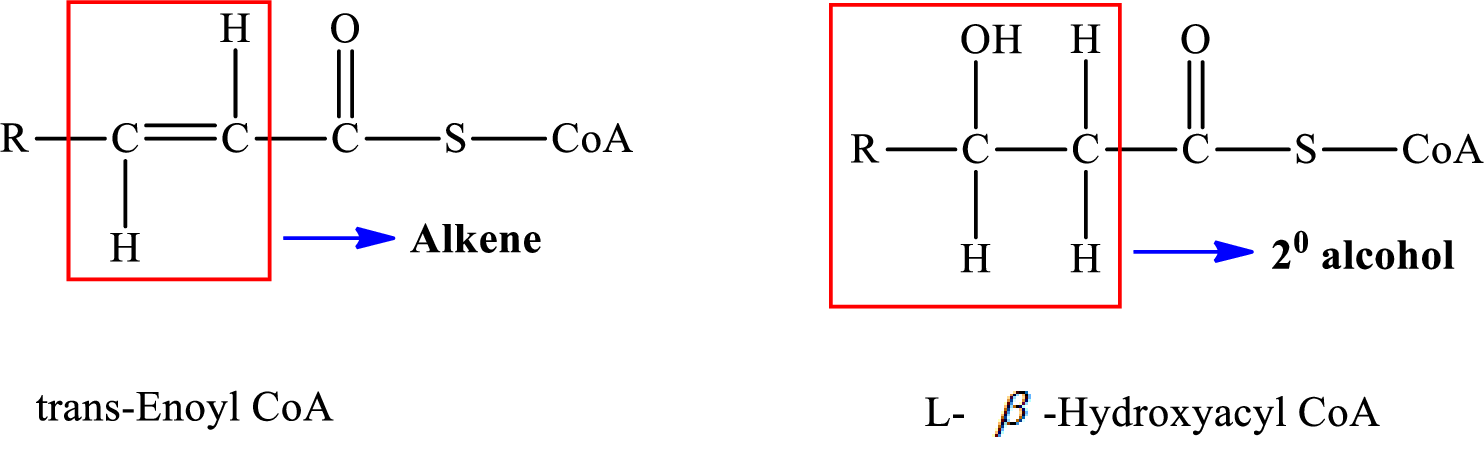
A double bond is present in
(c)
Interpretation: To determine the functional group change that occurs in step 3 of a turn of the
Concept introduction: The
Functional groups are defined as the group of atoms which are attached to the carbon backbone of organic compounds. These are generally heteroatoms which are attached to the parent hydrocarbon chain. Some examples of functional groups are as follows:
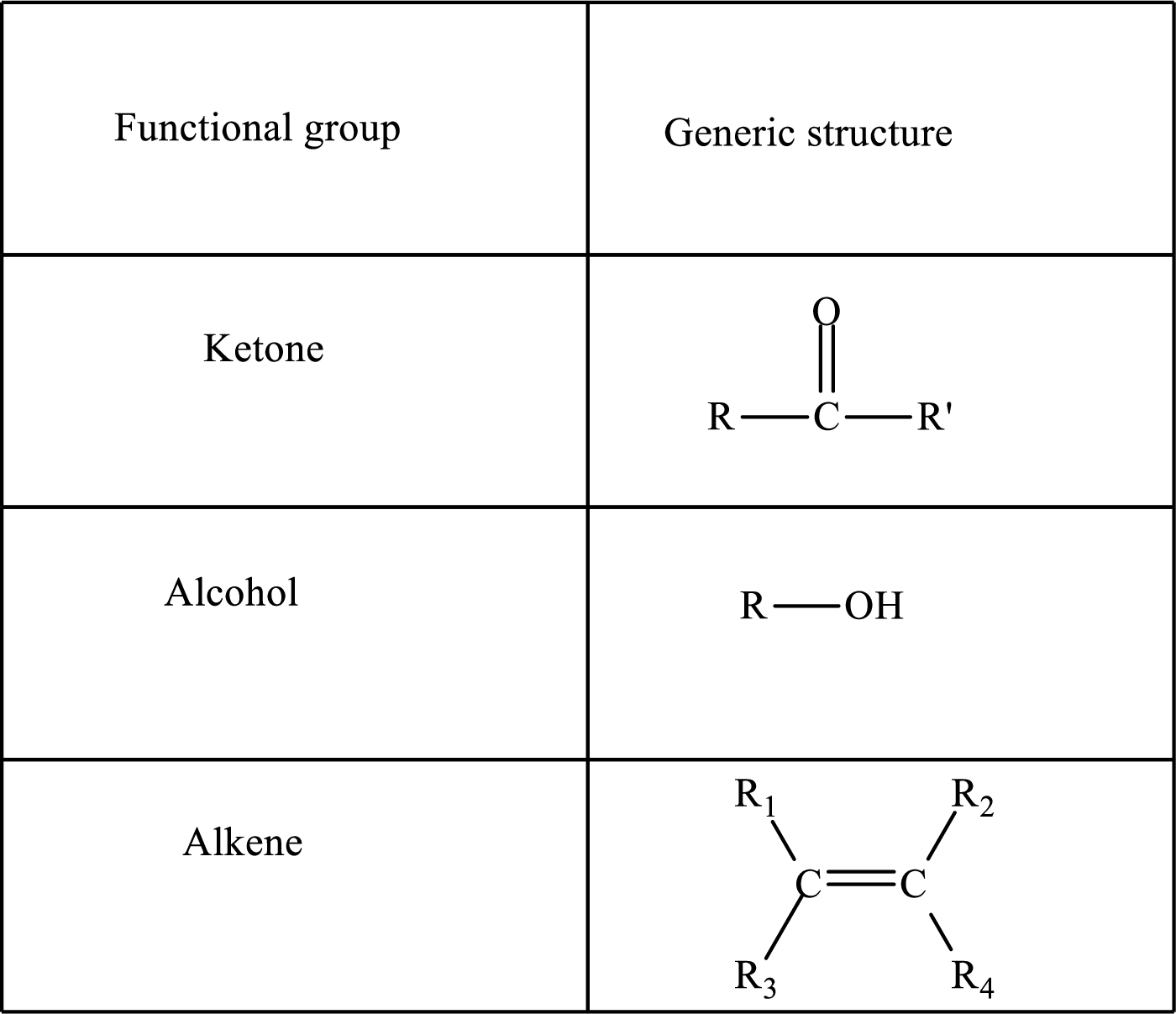
Here, R and
Alkanes are saturated hydrocarbons that contain covalently bonded hydrogen and carbon atoms. In secondary alcohol, the carbon atom of the hydroxyl group
(c)
Answer to Problem 25.29EP
In step 3 of a turn of the
Explanation of Solution
The reaction in step 3 of a turn of the
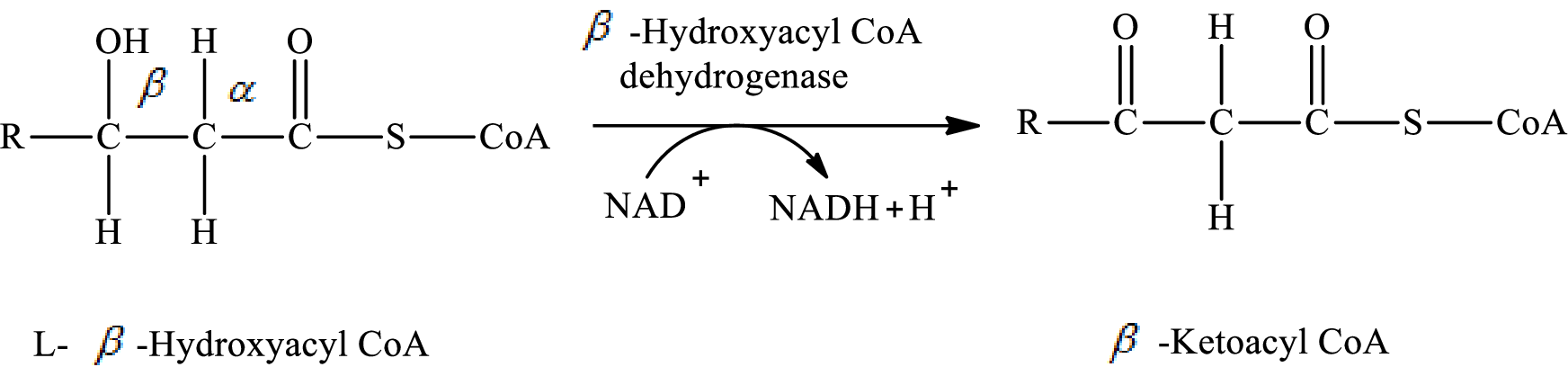
Therefore,
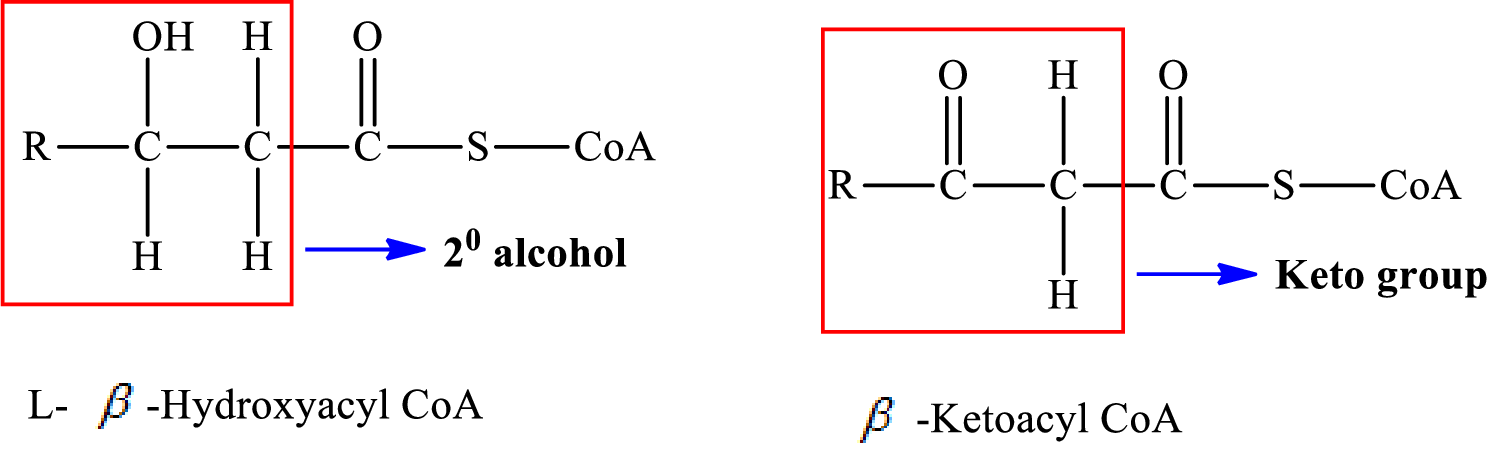
In
Want to see more full solutions like this?
Chapter 25 Solutions
General, Organic, and Biological Chemistry
- What type(s) of extremophiles are used in oil well drilling operations?arrow_forwardExplain why monitoring blood lactate levels might be a useful technique to gauge the amount of conditioning in an Olympic runner.arrow_forwardome athletes will consume only protein for several days before a competition, which reduces the amount of glycogen in both the muscle fibers and the liver. What impact would this have on their ability to perform activities that require short, powerful bursts of activity? How would it affect their ability to perform endurance activitiesarrow_forward
- Excess cortisol is actively binding to its receptors on the hypothalamus. The negative feedback mechanism of the endocrine system would cause which of the following? Question 9 options: A) increased cortisol production B) increased ACTH to be released into the blood stream C) inhibition of the production of ACTH and Cortisol Releasing Hormone D) nothing changesarrow_forwardRefer to the structure 1. The metabolic pathway is an example of a. Oxidative dealkylation b. Reduction c. Oxidative deamination d. Hydrolysis 2. This pathway is true for what kind of drugs a. Endogenous amides b. Endogenous amines c. Radical species d. Endogenous amino acidsarrow_forwardWhat is the point of having a number of enzymes activating each other, such as in the blood clotting cascade and other cascades? a. This slows the response to make it more manageable b. This produces a larger number of outcomes c. This uses the least amount of energy d. This greatly improves the response in both time and effectarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning
Chemistry In FocusChemistryISBN:9781305084476Author:Tro, Nivaldo J., Neu, Don.Publisher:Cengage Learning




