
(a)
Interpretation: The product formed by the treatment of given compound with
Concept introduction: Primary and secondary
Answer to Problem 25.29P
The product formed by the treatment of given compound with
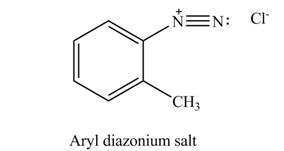
Explanation of Solution
Primary and secondary amines on reaction with
In the given compound, nitrogen atom is substituted by two hydrogen atoms and one phenyl group. Hence, it is a primary amine. Therefore, the product formed by the reaction of given compound with
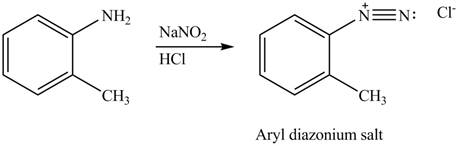
Figure 1
The product formed by the treatment of given compound with
(b)
Interpretation: The product formed by the treatment of given compound with
Concept introduction: Primary and secondary amines on reaction with
Answer to Problem 25.29P
The product formed by the treatment of given compound with
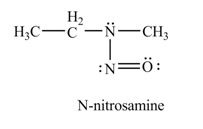
Explanation of Solution
Primary and secondary amines on reaction with
In the given compound, nitrogen atom is substituted by one hydrogen atom and two alkyl groups. Hence, it is a secondary amine. Therefore, the product formed by the reaction of given compound with
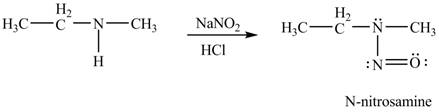
Figure 2
The product formed by the treatment of given compound with
(c)
Interpretation: The product formed by the treatment of given compound with
Concept introduction: Primary and secondary amines on reaction with
Answer to Problem 25.29P
The product formed by the treatment of given compound with
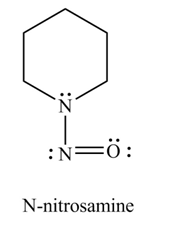
Explanation of Solution
Primary and secondary amines on reaction with
In the given compound, nitrogen atom is substituted by one hydrogen atom and two alkyl groups. Hence, it is a secondary amine. Therefore, the product formed by the reaction of given compound with
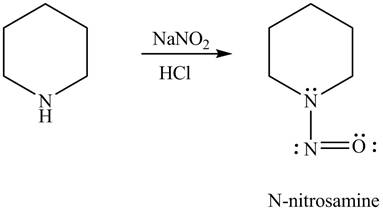
Figure 3
The product formed by the treatment of given compound with
(d)
Interpretation: The product formed by the treatment of given compound with
Concept introduction: Primary and secondary amines on reaction with
Answer to Problem 25.29P
The product formed by the treatment of given compound with
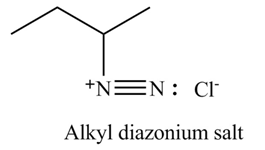
Explanation of Solution
Primary and secondary amines on reaction with
In the given compound, nitrogen atom is substituted by two hydrogen atoms and one alkyl group. Hence, it is a primary amine. Therefore, the product formed by the reaction of given compound with
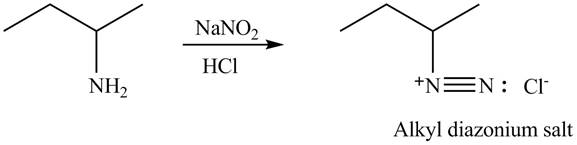
Figure 4
The product formed by the treatment of given compound with
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry
- What carbonyl compound and diol are needed to prepare each compound?arrow_forwardWhat product is formed when a solution of A to B is treated with mild base? This reaction is the first step in the synthesis of rosuvastatin (sold as a calcium salt under the trade name Crestor), a drug used to treat patients with high cholesterol.arrow_forward(a) Give an acceptable name for each compound, (b) Draw the organic products formed when A or B is treated with each reagent: [1] H3O+; [2] −OH, H2O; [3] CH3CH2CH2MgBr (excess), then H2O; [4] LiAlH4, then H2O.arrow_forward
- Ethers are oxidized with O2 to form hydroperoxides that decompose violently when heated. Draw a stepwise mechanism for this reaction.arrow_forwardWhat carbonyl compound and amine or alcohol are needed to prepare each product?arrow_forward(a) Give the IUPAC name for A and B. (b) Draw the product formed when A or B is treated with each reagent: [1] NaBH4, CH3OH; [2] CH3MgBr, then H2O; [3] Ph3P = CHOCH3; [4] CH3CH2CH2NH2, mild acid; [5] HOCH2CH2CH2OH, H+.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





