
Concept explainers
Draw the organic products formed in each reaction.
a. 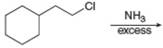 f.
f. 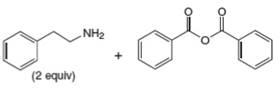
b. 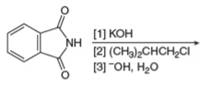 g.
g. 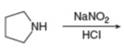
c. 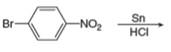 h.
h. 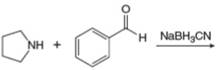
d.  i.
i. 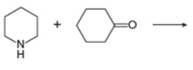
e. 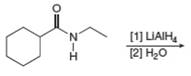 j.
j. 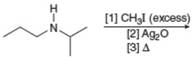
(a)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: The reaction between amine and alkyl halide is direct nucleophilic substitution reaction. The amino group acts as nucleophile and attacks the electrophilic carbon in alkyl halide followed by deprotonation to form amine or ammonium salt.
Answer to Problem 25.55P
The organic product formed in the given reaction is,
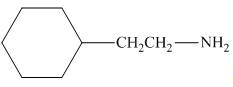
Figure 1
Explanation of Solution
The reaction between (
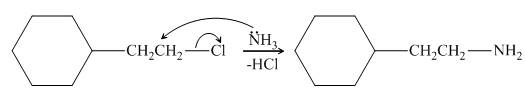
Figure 2
In the given reaction, the ammonia acts as nucleophile and attacks the carbon atom bonded to chlorine atom to produce the
The organic product formed in the given reaction is shown in Figure 1.
(b)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: Gabriel synthesis is a method of preparation of primary amines. This is a nucleophilic substitution reaction. The phthalimide in presence of base forms the resonance stabilized anion which acts as a nucleophile and attacks the electrophilic carbon of alkyl halides.
Answer to Problem 25.55P
The organic product formed in the given reaction is
Explanation of Solution
The reaction between
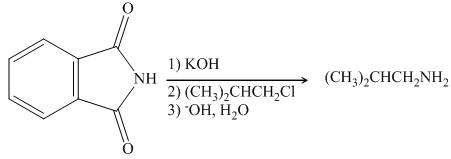
Figure 3
In presence of base, phthalimide forms the resonance stabilized anion which acts as a nucleophile and attacks the electrophilic carbon of
The organic product formed in the given reaction is
(c)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: The synthesis of amine can be carried out by reduction of nitro compounds, nitriles and amides. Nitro compounds can be reduced to amines using catalytic hydrogenation or iron/palladium/tin metal in presence of acid. Nitriles and amides are reduced to amines using lithium aluminium hydride.
Answer to Problem 25.55P
The organic product formed in the given reaction is,
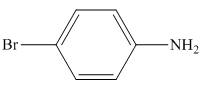
Figure 4
Explanation of Solution
In the presence of tin metal with acid,
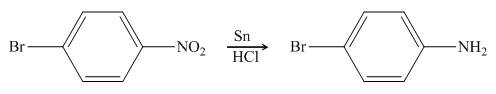
Figure 5
The organic product formed in the given reaction is shown in Figure 4.
(d)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: The synthesis of amine can be carried out by reduction of nitro compounds, nitriles and amides. Nitro compounds can be reduced to amines using catalytic hydrogenation or iron/palladium/tin metal in presence of acid. Nitriles and amides are reduced to amines using lithium aluminium hydride.
Answer to Problem 25.55P
The organic product formed in the given reaction is,
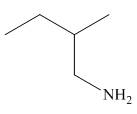
Figure 6
Explanation of Solution
In the presence of lithium aluminium hydride,
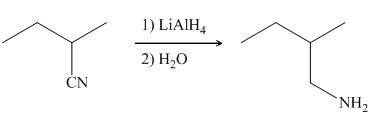
Figure 7
The organic product formed in the given reaction is shown in Figure 6.
(e)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: The synthesis of amine can be carried out by reduction of nitro compounds, nitriles and amides. Nitro compounds can be reduced to amines using catalytic hydrogenation or iron/palladium/tin metal in presence of acid. Nitriles and amides are reduced to amines using lithium aluminium hydride.
Answer to Problem 25.55P
The organic product formed in the given reaction is,
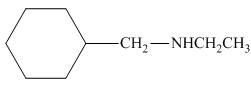
Figure 8
Explanation of Solution
In the presence of lithium aluminium hydride,
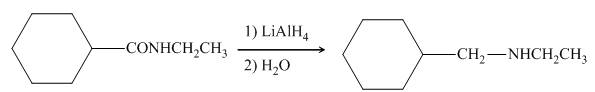
Figure 9
The organic product formed in the given reaction is shown in Figure 8.
(f)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: Amines reacts with acid chlorides or anhydrides to form amides. The amino group acts as nucleophile and attacks the carbonyl carbon of acid chlorides or anhydrides resulting in formation of amides and loss of chlorides or carboxylate ion.
Answer to Problem 25.55P
The organic product formed in the given reaction is,
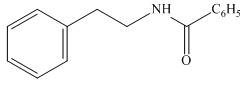
Figure 10
Explanation of Solution
The given amine,
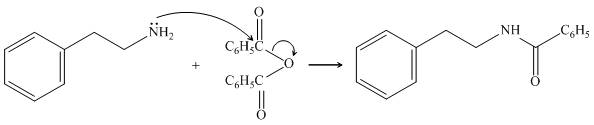
Figure 11
The organic product formed in the given reaction is shown in Figure 10.
(g)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: Sodium nitrite and hydrochloric acid reacts to form nitrous acid. Nitrous acid in presence of acid forms nitrosonium ion which reacts with primary amines to produce diazonium salt and with secondary amines produce
Answer to Problem 25.55P
The organic product formed in the given reaction is,
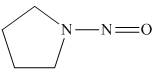
Figure 12
Explanation of Solution
Pyrrolidine reacts with nitrous acid to form
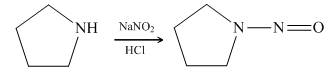
Figure 13
The organic product formed in the given reaction is shown in Figure 12.
(h)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: The conversion of aldehydes and ketones into amines is known as reductive amination. The first step is the formation of imines using aldehydes or ketones and amines. The second step involves the reduction of imine to form amine. The best reagent for this reaction is sodiumcyanoborohydride.
Answer to Problem 25.55P
The organic product formed in the given reaction is,
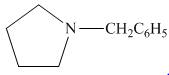
Figure 14
Explanation of Solution
Pyrrolidine reacts with benzaldehyde to form imine. The imine undergoes reduction to form
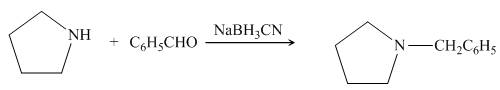
Figure 15
The organic product formed in the given reaction is shown in Figure 14.
(i)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: Aldehyde or ketone on reaction with primary amine forms imine. Imine is also known as Schiff base. The nucleophilic attack of primary amine on carbonyl carbon results in the formation of intermediate carbinolamine. This intermediate loses water to form an imine.
Answer to Problem 25.55P
The organic product formed in the given reaction is,
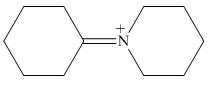
Figure 16
Explanation of Solution
Piperidine reacts with cyclohexanone to form imine. The corresponding reaction is shown below.
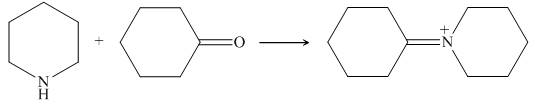
Figure 17
The organic product formed in the given reaction is shown in Figure 16.
(j)
Interpretation: The organic product formed in the given reaction is to be drawn.
Concept introduction: The conversion of amines into alkenes can be achieved by Hoffmann elimination reaction. Amines have poor leaving groups. They react with excess of alkyl halides to form ammonium salts. These ammonium salts undergo
Answer to Problem 25.55P
The organic product formed in the given reaction is,
![]()
Figure 18
Explanation of Solution
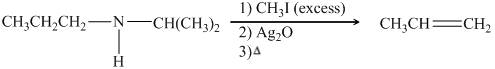
Figure 19
The organic product formed in the given reaction is shown in Figure 18.
Want to see more full solutions like this?
Chapter 25 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





