
Concept explainers
(a)
Interpretation:
The structural formula for
Concept introduction:
The formula which represents the bonding and type of bonds that hold the atoms in a molecule together is said to be a structural formula.
Answer to Problem 1E
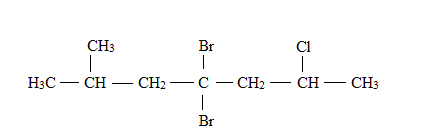
Explanation of Solution
There is a difference between structural formula and condensed formula.
The formula which represents the bonding and type of bonds that hold the atoms in a molecule together is said to be a structural formula.
In condensed structural formula, the organic structures of the compound are written in a line of text showing all the atoms in the molecule and omitting the vertical and horizontal bonds.
The structural formula for
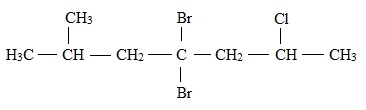
(b)
Interpretation:
The structural formula for
Concept introduction:
The formula which represents the bonding and type of bonds that hold the atoms in a molecule together is said to be a structural formula.
Answer to Problem 1E
Explanation of Solution
There is a difference between structural formula and condensed formula.
The formula which represents the bonding and type of bonds that hold the atoms in a molecule together is said to be a structural formula.
In condensed structural formula, the organic structures of the compound are written in a line of text showing all the atoms in the molecule and omitting the vertical and horizontal bonds.
The structural formula for
(c)
Interpretation:
The structural formula for
Concept introduction:
The formula which represents the bonding and type of bonds that hold the atoms in a molecule together is said to be a structural formula.
Answer to Problem 1E
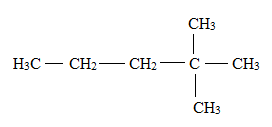
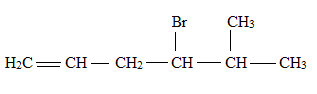
Explanation of Solution
There is a difference between the structural formula and the condensed formula.
The formula which represents the bonding and the type of bonds that hold the atoms in a molecule together is said to be a structural formula.
In condensed structural formula, the organic structures of the compound are written in a line of text showing all the atoms in the molecule and omitting the vertical and horizontal bonds.
The structural formula for
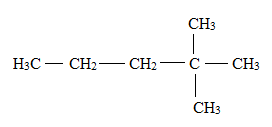
(d)
Interpretation:
The structural formula for
Concept introduction:
The formula which represents the bonding and the type of bonds that hold the atoms in a molecule together is said to be a structural formula.
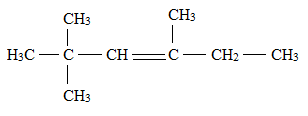
Answer to Problem 1E
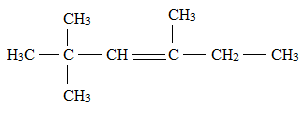
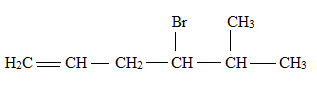
Explanation of Solution
There is a difference between a structural formula and a condensed formula.
The formula which represents the bonding and type of bonds that hold the atoms in a molecule together is said to be a structural formula.
In condensed structural formula, the organic structures of the compound are written in a line of text showing all the atoms in the molecule and omitting the vertical and horizontal bonds.
The structural formula for
Want to see more full solutions like this?
Chapter 26 Solutions
General Chemistry: Principles and Modern Applications - With Solutions Manual and Modified MasteringChemistry Code
- What is the meaning of the term tertiary (3) when it is used to classify alcohols? Draw a structural formula for the one tertiary (3) alcohol with the molecular formula C4H10O.arrow_forwardConsider the compound C₂H₃N. Which one of the structures in Figure 4 is the best representation of this compound based on your current knowledge? * A B C D All these structures are good representations of the compound.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning





