
Concept explainers
Draw the products formed in each reaction.
a. 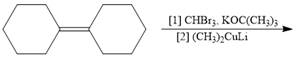 e.
e. 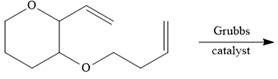
b. 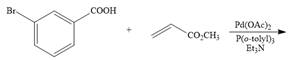 f.
f. 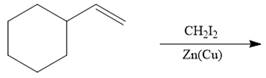
c. 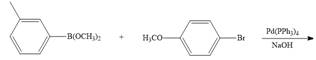 g.
g. 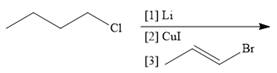
d. 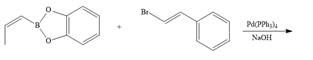 h.
h. 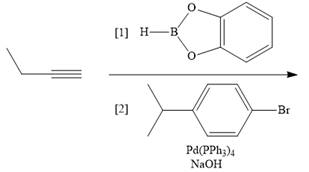
(a)
Interpretation: The product formed in the given reaction is to be drawn.
Concept introduction: The treatment of
Answer to Problem 26.34P
The product of the given reaction is,
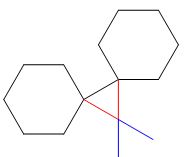
Explanation of Solution
The given reaction involves treatment of an alkene with
The treatment of
The corresponding reaction is shown below.
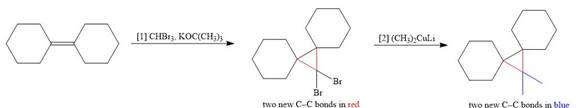
Figure 1
Thus, the product of the given reaction is,

Figure 2
The product of the given reaction is drawn in Figure 2.
(b)
Interpretation: The product formed in the given reaction is to be drawn.
Concept introduction: The treatment of an organic halide with an alkene in the presence of
Answer to Problem 26.34P
The product of the given reaction is,
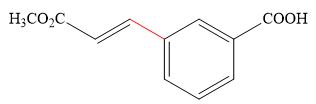
Explanation of Solution
The given reaction involves treatment of an organic halide with an alkene in the presence of
The treatment of an organic halide with an alkene in the presence of
The corresponding reaction is shown below.
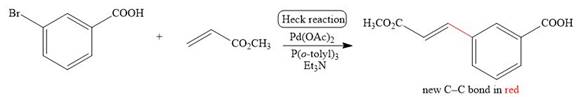
Figure 3
Thus, the product of the given reaction is,

Figure 4
The product of the given reaction is drawn in Figure 4.
(c)
Interpretation: The product formed in the given reaction is to be drawn.
Concept introduction: The treatment of an organic halide
Answer to Problem 26.34P
The product of the given reaction is,
Explanation of Solution
The given reaction involves treatment of an organic halide with an organoborane reagent in the presence of
The treatment of an organic halide
The corresponding reaction is shown below.
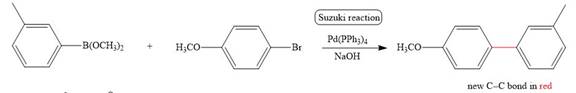
Figure 5
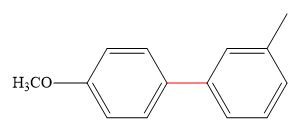
Thus, the product of the given reaction is,

Figure 6
The product of the given reaction is drawn in Figure 6.
(d)
Interpretation: The product formed in the given reaction is to be drawn.
Concept introduction: The treatment of an organic halide
Answer to Problem 26.34P
The product of the given reaction is,
Explanation of Solution
The given reaction involves treatment of an organic halide with an organoborane reagent in the presence of
The treatment of an organic halide
The corresponding reaction is shown below.
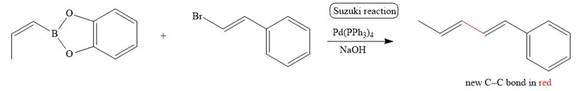
Figure 7
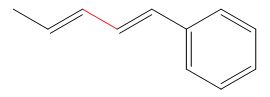
Thus, the product of the given reaction is,

Figure 8
The product of the given reaction is drawn in Figure 8.
(e)
Interpretation: The product formed in the given reaction is to be drawn.
Concept introduction: The ring-closing metathesis (RCM) by Grubbs catalyst occurs, when the starting material is diene. This reaction is facilitated under high-dilution condition as it favors intramolecular metathesis instead of intermolecular metathesis.
Answer to Problem 26.34P
The product of the given reaction is,
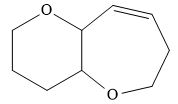
Explanation of Solution
The given reaction involves treatment of a diene with Grubbs catalyst.
The ring-closing metathesis (RCM) by Grubbs catalyst occurs, when the starting material is diene. This reaction is facilitated under high-dilution condition as it favors intramolecular metathesis instead of intermolecular metathesis.
The corresponding reaction is shown below.
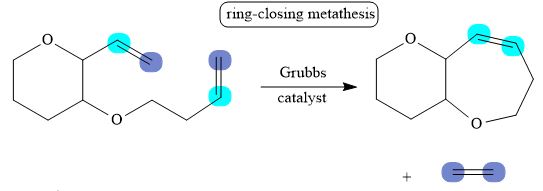
Figure 9
Thus, the product of the given reaction is,

Figure 10
The product of the given reaction is drawn in Figure 10.
(f)
Interpretation: The product formed in the given reaction is to be drawn.
Concept introduction: The nonhalogenated cyclopropanes are synthesized by the treatment of an alkene with
Answer to Problem 26.34P
The product of the given reaction is,
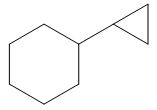
Explanation of Solution
The given reaction involves treatment of an alkene with
The nonhalogenated cyclopropanes are synthesized by the treatment of an alkene with
The corresponding reaction is shown below.
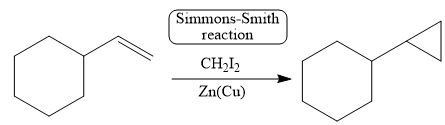
Figure 11
Thus, the product of the given reaction is,

Figure 12
The product of the given reaction is drawn in Figure 12.
(g)
Interpretation: The product formed in the given reaction is to be drawn.
Concept introduction: The treatment of an organic halide with
Answer to Problem 26.34P
The product of the given reaction is,
![]()
Explanation of Solution
The given reaction involves treatment of chlorobutane with
The treatment of an organic halide with
The corresponding reaction is shown below.
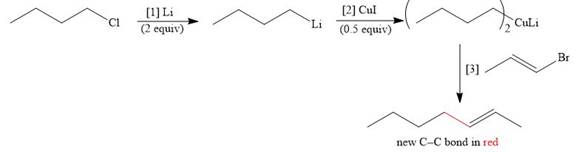
Figure 13
Thus, the product of the given reaction is,
![]()
Figure 14
The product of the given reaction is drawn in Figure 14.
(h)
Interpretation: The product formed in the given reaction is to be drawn.
Concept introduction: The treatment of an organic halide
Answer to Problem 26.34P
The product of the given reaction is,
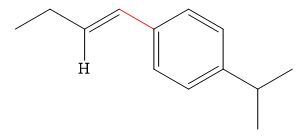
Explanation of Solution
The given reaction involves treatment of an alkyne with an vinylborane, followed by treatment with organic halide in the presence of
The treatment of given alkyne with catecholborane leads to the formation of corresponding vinylborane in first step. This vinylborane (an organoborane) is formed by hydroboration which adds
The treatment of an organic halide
The corresponding reaction is shown below.
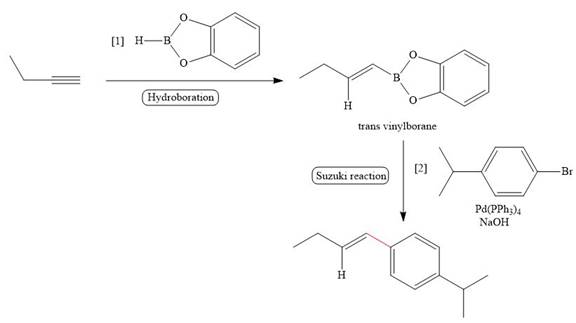
Figure 15
Thus, the product of the given reaction is,

Figure 16
The product of the given reaction is drawn in Figure 16.
Want to see more full solutions like this?
Chapter 26 Solutions
Organic Chemistry
Additional Science Textbook Solutions
Basic Chemistry (5th Edition)
Organic Chemistry As a Second Language: Second Semester Topics
General, Organic, and Biological Chemistry - 4th edition
Chemistry For Changing Times (14th Edition)
General, Organic, and Biological Chemistry (3rd Edition)
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

