
EBK ORGANIC CHEMISTRY
10th Edition
ISBN: 9781259636875
Author: Carey
Publisher: MCGRAW HILL BOOK COMPANY
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 27, Problem 15P
Interpretation Introduction
Interpretation: The structure of 5-Fluorouracil has to be drawn.
Concept introduction: The compound 5-fluorouracil is used in the chemotherapy of breast-cancer. Uracil belongs to the class of pyrimidines. It is a nucleobase commonly found in RNA.
Expert Solution & Answer
Answer to Problem 15P
Solution:
The structure of 5-fluorouracil is shown below.
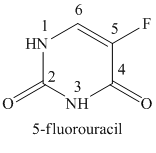
Explanation of Solution
The structure of uracil is shown below.
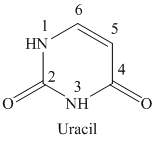
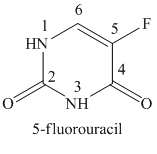
If a fluoro substituent is attached to the fifth position of the uracil ring, then the structure obtained is called 5-fluorouracil. The structure of 5-fluorouracil is shown below.
Conclusion
In the structure of 5-Fluorouracil, a fluoro substituent is attached to the fifth position of the uracil ring.
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
6. Aldehydes are characterized by reactions:
A) Nucleophilic addition of amines
B) Nucleophilic addition of water
C) Nucleophilic addition of alcohols
D) Polymerization
The reaction of 4-methylcyclohexanone with CH3MgBr followed by neutralization gives two alcohols. These two alcohols are
A.
enantiomers formed in equal amounts.
B.
diastereomers.
C.
constitutional isomers.
D.
enantiomers formed in unequal amounts.
Aziridines are heterocycles that contain an N atom in a three-membered ring. Like epoxides, aziridines are strained and reactive because the 60° bond angles of the three-membered ring deviate greatly from the theoretical tetrahedral bond angle. One step in the synthesis of the drug oseltamivir (trade name Tamiu, Section 3.2) involves the conversion of amine X to diamine Y, a reaction that occurs by way of an intermediate aziridine. Draw a stepwise mechanism for the conversion of X to Y. Indicate the structure of the aziridine intermediate, and explain the trans stereochemistry of the two amines in Y.
Chapter 27 Solutions
EBK ORGANIC CHEMISTRY
Ch. 27.1 - Problem 27.1 Write a structural formula for the...Ch. 27.1 - Prob. 2PCh. 27.1 - Prob. 3PCh. 27.2 - Prob. 4PCh. 27.3 - Prob. 5PCh. 27.3 - Prob. 6PCh. 27.5 - Prob. 7PCh. 27.5 - Prob. 8PCh. 27.5 - Prob. 9PCh. 27.6 - Prob. 10P
Ch. 27.7 - Prob. 11PCh. 27.9 - Prob. 12PCh. 27.12 - 27.13 Modify Figure 27.12 so that it corresponds...Ch. 27.13 - Prob. 14PCh. 27 - Prob. 15PCh. 27 - Prob. 16PCh. 27 - Prob. 17PCh. 27 - Nebularine is a toxic nucleoside isolated from a...Ch. 27 - Prob. 19PCh. 27 - The 5-nucleotide of inosine, inosinic acid...Ch. 27 - Prob. 21PCh. 27 - (a) The two most acidic hydrogens of uracil have...Ch. 27 - The phosphorylation of -D-glucopyranose by ATP...Ch. 27 - When 6-chloropurine is heated with aqueous sodium...Ch. 27 - Prob. 25PCh. 27 - Prob. 26PCh. 27 - Prob. 27PCh. 27 - Prob. 28PCh. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Aziridines are heterocycles that contain an N atom in a three-membered ring. Like epoxides, aziridines are strained and reactive because the 60° bond angles of the three-membered ring deviate greatly from the theoretical tetrahedral bond angle. One step in the synthesis of the drug oseltamivir (trade name Tamiflu, Section 3.2) involves the conversion of amine X to diamine Y, a reaction that occurs by way of an intermediate aziridine. Draw a stepwise mechanism for the conversion of X to Y. Indicate the structure of the aziridine intermediate, and explain the trans stereochemistry of the two amines in Y.arrow_forwardThe shrub ma huang (Section 5.4A) contains two biologically activestereoisomers—ephedrine and pseudoephedrine—with two stereogeniccenters as shown in the given structure. Ephedrine is one component ofa once-popular combination drug used by body builders to increaseenergy and alertness, whereas pseudoephedrine is a nasaldecongestant.a.) Draw the structure of naturally occurring (−)-ephedrine, which has the1R,2S configuration.b.) Draw the structure of naturally occurring (+)-pseudoephedrine, whichhas the 1S,2S configuration.c.) How are ephedrine and pseudoephedrine related?d.) Draw all other stereoisomers of (−)-ephedrine and (+) pseudoephedrine, and give the R,S designation for all stereogeniccenters.e.) How is each compound drawn in part (d) related to (−)-ephedrine?arrow_forwardGive the IUPAC names (including E,Z and R,S designations).arrow_forward
- Several sulfonylureas, a class of compounds containing RSO2NHCONHR, are useful drugs as orally active replacements for injected insulin in patients with adult-onset diabetes. These drugs decrease blood glucose concentrations by stimulating b cells of the pancreas to release insulin and by increasing the sensitivity of insulin receptors in peripheral tissues to insulin stimulation. Tolbutamide is synthesized by the reaction of the sodium salt of p-toluenesulfonamide and ethyl N-butylcarbamate . Propose a mechanism for this step.arrow_forwardWhat bonds are affected by the following denaturation agents; a) Heat b) Organic solvents c) Metallic salts d) Alkaloidal reagents e) Strong acidsarrow_forward2. What is the organic by-product in a haloform reaction? A) an ether B) a haloform C) an alcohol D) an ester E) an amidearrow_forward
- How can pentan-2-one be converted to each compound?arrow_forwardEleostearic acid, C18H30O2, is a rare fatty acid found in the tung oil used for finishing furniture. On ozonolysis followed by treatment with zinc, eleostearic acid furnishes one part pentanal, two parts glyoxal (OHC-CHO), and one part 9-oxononanoic acid [OHC(CH2)7CO2H]. What is the structure of eleostearic acid?arrow_forwardComplete A-> H for this reaction by fill in the blank with appropriate chemicalsarrow_forward
- Draw the major product of the reaction between 1-butanol and Na2Cr2O7, H2SO4, H2O.arrow_forwardThe Ka1 of ascorbic acid is 7.94 x 10-5. Would you expect ascorbic acid dissolved in blood plasma (pH 7.35–7.45) to exist primarily as ascorbic acid or as ascorbate anion? Explain.arrow_forwardComplete the reaction: 42K19 → + β + [a]_____________arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY