
Organic And Biological Chemistry
7th Edition
ISBN: 9781305081079
Author: STOKER, H. Stephen (howard Stephen)
Publisher: Cengage Learning,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2.7, Problem 2QQ
Interpretation Introduction
Interpretation:
The number of carbon atoms present in terpene is always a multiple of how many number of carbon atoms has to be chosen from the given options.
Concept Introduction:
Terpenes are organic compounds. The carbon skeleton of terpene is made up of two or more 5-carbon isoprene structural units.
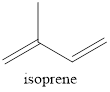
2-methyl-1,3-butadiene is known as isoprene. Isoprene is the five‑carbon diene. The structure of isoprene can be given as,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
For which of the following compounds does 1.9g represent 4.3*10-2 moles of the compound?
A) C3H8
B) CO2
C) H2O2
D) no correct response
E) more than one correct response
For which of the following halogenated cycloalkanes is cis-trans isomerism possible?
a) 1,1-dichlorocyclobutane
b) 1-bromo-1-chlorocyclobutane
c) 1-bromo-2-chlorocyclobutane
d) more than one correct response
Chapter 2 Solutions
Organic And Biological Chemistry
Ch. 2.1 - Prob. 1QQCh. 2.1 - Prob. 2QQCh. 2.1 - Prob. 3QQCh. 2.2 - Prob. 1QQCh. 2.2 - Prob. 2QQCh. 2.2 - Prob. 3QQCh. 2.2 - Prob. 4QQCh. 2.3 - Prob. 1QQCh. 2.3 - Prob. 2QQCh. 2.3 - Prob. 3QQ
Ch. 2.3 - Prob. 4QQCh. 2.4 - Prob. 1QQCh. 2.4 - Prob. 2QQCh. 2.5 - Prob. 1QQCh. 2.5 - Prob. 2QQCh. 2.5 - Prob. 3QQCh. 2.6 - Prob. 1QQCh. 2.6 - Prob. 2QQCh. 2.6 - Prob. 3QQCh. 2.7 - Prob. 1QQCh. 2.7 - Prob. 2QQCh. 2.7 - Prob. 3QQCh. 2.8 - Prob. 1QQCh. 2.8 - Prob. 2QQCh. 2.9 - Prob. 1QQCh. 2.9 - Prob. 2QQCh. 2.10 - Prob. 1QQCh. 2.10 - Prob. 2QQCh. 2.10 - Prob. 3QQCh. 2.10 - Prob. 4QQCh. 2.10 - Prob. 5QQCh. 2.11 - Prob. 1QQCh. 2.11 - Prob. 2QQCh. 2.11 - Prob. 3QQCh. 2.11 - Prob. 4QQCh. 2.11 - Prob. 5QQCh. 2.12 - Prob. 1QQCh. 2.12 - Prob. 2QQCh. 2.12 - Prob. 3QQCh. 2.12 - Prob. 4QQCh. 2.12 - Prob. 5QQCh. 2.13 - Prob. 1QQCh. 2.13 - Prob. 2QQCh. 2.13 - Prob. 3QQCh. 2.14 - Prob. 1QQCh. 2.14 - Prob. 2QQCh. 2.14 - Prob. 3QQCh. 2.14 - Prob. 4QQCh. 2.15 - Prob. 1QQCh. 2.15 - Prob. 2QQCh. 2.15 - Prob. 3QQCh. 2.15 - Prob. 4QQCh. 2.16 - Prob. 1QQCh. 2.16 - Prob. 2QQCh. 2 - Classify each of the following hydrocarbons as...Ch. 2 - Prob. 2.2EPCh. 2 - Prob. 2.3EPCh. 2 - Prob. 2.4EPCh. 2 - Prob. 2.5EPCh. 2 - Prob. 2.6EPCh. 2 - Prob. 2.7EPCh. 2 - Characterize the physical properties of saturated...Ch. 2 - Prob. 2.9EPCh. 2 - Prob. 2.10EPCh. 2 - Prob. 2.11EPCh. 2 - Prob. 2.12EPCh. 2 - Prob. 2.13EPCh. 2 - Prob. 2.14EPCh. 2 - What is the name of the spatial arrangement for...Ch. 2 - Prob. 2.16EPCh. 2 - Prob. 2.17EPCh. 2 - Prob. 2.18EPCh. 2 - Draw a condensed structural formula for each of...Ch. 2 - Prob. 2.20EPCh. 2 - The following names are incorrect by IUPAC rules....Ch. 2 - The following names are incorrect by IUPAC rules....Ch. 2 - Prob. 2.23EPCh. 2 - Draw a condensed structural formula for each of...Ch. 2 - Prob. 2.25EPCh. 2 - Classify each of the following compounds as...Ch. 2 - Prob. 2.27EPCh. 2 - How many hydrogen atoms are present in a molecule...Ch. 2 - Draw a line-angle structural formula for each of...Ch. 2 - Draw a line-angle structural formula for each of...Ch. 2 - Prob. 2.31EPCh. 2 - Prob. 2.32EPCh. 2 - Prob. 2.33EPCh. 2 - Prob. 2.34EPCh. 2 - Prob. 2.35EPCh. 2 - Prob. 2.36EPCh. 2 - Prob. 2.37EPCh. 2 - Prob. 2.38EPCh. 2 - For each of the following pairs of alkenes,...Ch. 2 - For each of the following pairs of alkenes,...Ch. 2 - Prob. 2.41EPCh. 2 - Prob. 2.42EPCh. 2 - Prob. 2.43EPCh. 2 - Prob. 2.44EPCh. 2 - Prob. 2.45EPCh. 2 - Prob. 2.46EPCh. 2 - For each molecule, indicate whether cistrans...Ch. 2 - Prob. 2.48EPCh. 2 - Prob. 2.49EPCh. 2 - Prob. 2.50EPCh. 2 - Draw a structural formula for each of the...Ch. 2 - Prob. 2.52EPCh. 2 - Prob. 2.53EPCh. 2 - For each of the following molecules, indicate...Ch. 2 - Prob. 2.55EPCh. 2 - Prob. 2.56EPCh. 2 - Prob. 2.57EPCh. 2 - Prob. 2.58EPCh. 2 - Prob. 2.59EPCh. 2 - How many isoprene units are present in a....Ch. 2 - Prob. 2.61EPCh. 2 - Indicate whether each of the following statements...Ch. 2 - Prob. 2.63EPCh. 2 - Prob. 2.64EPCh. 2 - Prob. 2.65EPCh. 2 - Prob. 2.66EPCh. 2 - Prob. 2.67EPCh. 2 - Prob. 2.68EPCh. 2 - Prob. 2.69EPCh. 2 - Prob. 2.70EPCh. 2 - Prob. 2.71EPCh. 2 - Prob. 2.72EPCh. 2 - Prob. 2.73EPCh. 2 - Prob. 2.74EPCh. 2 - Prob. 2.75EPCh. 2 - Prob. 2.76EPCh. 2 - Supply the structural formula of the product in...Ch. 2 - Prob. 2.78EPCh. 2 - Prob. 2.79EPCh. 2 - What reactant would you use to prepare each of the...Ch. 2 - Prob. 2.81EPCh. 2 - Prob. 2.82EPCh. 2 - Prob. 2.83EPCh. 2 - Prob. 2.84EPCh. 2 - Prob. 2.85EPCh. 2 - Prob. 2.86EPCh. 2 - Prob. 2.87EPCh. 2 - Prob. 2.88EPCh. 2 - Prob. 2.89EPCh. 2 - Prob. 2.90EPCh. 2 - Prob. 2.91EPCh. 2 - Prob. 2.92EPCh. 2 - Prob. 2.93EPCh. 2 - Prob. 2.94EPCh. 2 - Prob. 2.95EPCh. 2 - Prob. 2.96EPCh. 2 - Prob. 2.97EPCh. 2 - Prob. 2.98EPCh. 2 - Prob. 2.99EPCh. 2 - Prob. 2.100EPCh. 2 - Prob. 2.101EPCh. 2 - Prob. 2.102EPCh. 2 - Prob. 2.103EPCh. 2 - Prob. 2.104EPCh. 2 - Prob. 2.105EPCh. 2 - Prob. 2.106EPCh. 2 - Prob. 2.107EPCh. 2 - Prob. 2.108EPCh. 2 - Assign each of the compounds in Problem 13-107 an...Ch. 2 - Assign each of the compounds in Problem 13-108 an...Ch. 2 - Prob. 2.111EPCh. 2 - Prob. 2.112EPCh. 2 - Prob. 2.113EPCh. 2 - Prob. 2.114EPCh. 2 - Prob. 2.115EPCh. 2 - Prob. 2.116EPCh. 2 - Prob. 2.117EPCh. 2 - Prob. 2.118EPCh. 2 - Prob. 2.119EPCh. 2 - Prob. 2.120EPCh. 2 - Prob. 2.121EPCh. 2 - Prob. 2.122EPCh. 2 - Prob. 2.123EPCh. 2 - Prob. 2.124EPCh. 2 - Prob. 2.125EPCh. 2 - Prob. 2.126EPCh. 2 - Prob. 2.127EPCh. 2 - Prob. 2.128EPCh. 2 - Prob. 2.129EPCh. 2 - Prob. 2.130EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,