
Concept explainers
(a)
Interpretation:
The curved arrow mechanism for the reaction is to be stated and the reaction is to be classified as electrocyclic, cycloaddition, and the sigmatropic reaction. Along with that the number of electrons involved in the reaction is to be stated.
Concept introduction:
In the pericyclic reactions, the bond formation and dissociation occur simultaneously. The pericyclic reaction occurs in a concerted fashion with the cyclic shift of electrons. They are of three types, electrocyclic, cycloaddition and the sigmatropic reactions. They occur in the presence of heat or light.
Answer to Problem 28.1P
The given reaction is an example of an electrocyclic pericyclic reaction. The number of electrons involved in this reaction is six. The curved arrow mechanism is shown below.
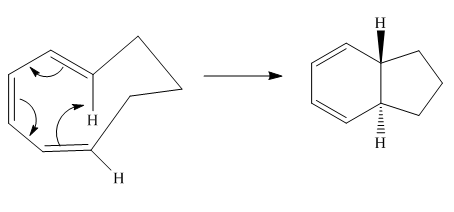
Explanation of Solution
The given reaction is an example of an electrocyclic reaction. This is because of in this electrocyclic reaction there is an intramolecular

Figure 1
The curved arrow mechanism for the reaction is shown in Figure 1. The reaction is a type of electrocyclic reaction. Along with it, the number of electrons involved in this reaction is six.
(b)
Interpretation:
The curved arrow mechanism for the reaction is to be stated and the reaction is to be classified as electrocyclic, cycloaddition, and the sigmatropic reaction. Along with that the number of electrons involved in the reaction is to be stated.
Concept introduction:
In the pericyclic reactions, the bond formation and dissociation occur simultaneously. The pericyclic reaction occurs in a concerted fashion with the cyclic shift of electrons. They are of three types electrocyclic, cycloaddition and the sigmatropic reactions. They occur in the presence of heat or light.
Answer to Problem 28.1P
The given reaction is an example of a sigmatropic pericyclic reaction. The number of electrons involved in this reaction is two. The curved arrow mechanism is shown below.
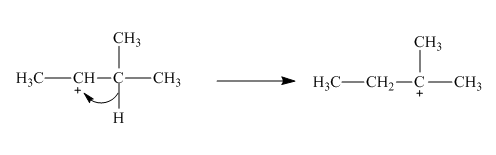
Explanation of Solution
The given reaction is an example of a sigmatropic reaction. This is because of in this sigmatropic reaction the allylic sigma bond present at one end of the system migrate to the other end of the system. The number of

Figure 2
The curved arrow mechanism for the reaction is shown in Figure 2. The reaction is a type of sigmatropic reaction. Along with it, the number of electrons involved in this reaction is two.
(c)
Interpretation:
The curved arrow mechanism for the reaction is to be stated and the reaction is to be classified as electrocyclic, cycloaddition, and the sigmatropic reaction. Along with that the number of electrons involved in the reaction is to be stated.
Concept introduction:
In the pericyclic reactions, the bond formation and dissociation occur simultaneously. The pericyclic reaction occurs in a concerted fashion with the cyclic shift of electrons. They are of three types electrocyclic, cycloaddition and the sigmatropic reactions. They occur in the presence of heat or light.
Answer to Problem 28.1P
The given reaction is an example of a cycloaddition pericyclic reaction. The number of electrons involved in this reaction is four. The curved arrow mechanism is shown below.
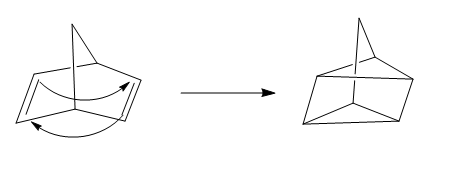
Explanation of Solution
The given reaction is an example of a cycloaddition reaction. This is because of in this cycloaddition reaction two

Figure 3
The curved arrow mechanism for the reaction is shown in Figure 3. The reaction is a type of cycloaddition reaction. Along with it, the number of electrons involved in this reaction is four.
(d)
Interpretation:
The curved arrow mechanism for the reaction is to be stated and the reaction is to be classified as electrocyclic, cycloaddition, and the sigmatropic reaction. Along with that the number of electrons involved in the reaction is to be stated.
Concept introduction:
In the pericyclic reactions, the bond formation and dissociation occur simultaneously. The pericyclic reaction occurs in a concerted fashion with the cyclic shift of electrons. They are of three types electrocyclic, cycloaddition and the sigmatropic reactions. They occur in the presence of heat or light.
Answer to Problem 28.1P
The given reaction is an example of a sigmatropic pericyclic reaction. The number of electrons involved in this reaction is six. The curved arrow mechanism is shown below.
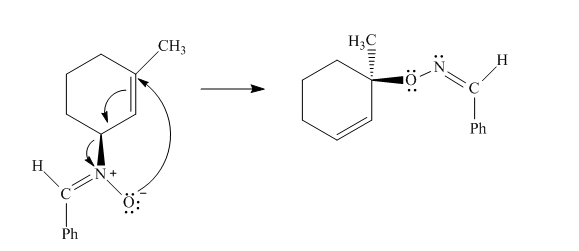
Explanation of Solution
The given reaction is an example of a sigmatropic reaction. This is because of in this sigmatropic reaction the allylic sigma bond present at one end of the

Figure 4
The curved arrow mechanism for the reaction is shown in Figure 4. The reaction is a type of sigmatropic reaction. Along with it, the number of electrons involved in this reaction is six.
(e)
Interpretation:
The curved arrow mechanism for the reaction is to be stated and the reaction is to be classified as electrocyclic, cycloaddition, and the sigmatropic reaction. Along with that the number of electrons involved in the reaction is to be stated.
Concept introduction:
In the pericyclic reactions, the bond formation and dissociation occur simultaneously. The pericyclic reaction occurs in a concerted fashion with the cyclic shift of electrons. They are of three types electrocyclic, cycloaddition and the sigmatropic reactions. They occur in the presence of heat or light.
Answer to Problem 28.1P
The given reaction is an example of a retro-electrocyclic pericyclic reaction. The number of electrons involved in this reaction is two. The curved arrow mechanism is shown below.
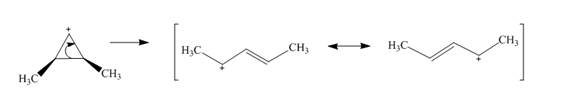
Explanation of Solution
The given reaction is an example of a retro-electrocyclic reaction. This is because of in the electrocyclic reaction there is an intramolecular

Figure 5
The curved arrow mechanism for the reaction is shown in Figure 5. The reaction is a type of retro-electrocyclic reaction. Along with it, the number of electrons involved in this reaction is two.
Want to see more full solutions like this?
Chapter 28 Solutions
Organic Chemistry
- Name the kind of sigmatropic rearrangement that occurs in each of the following reactions. a. Using arrows, show the electron rearrangement that takes place in each reaction.arrow_forwardFor the following reactions:a. Provide the missing major product, OR, missing reagent and conditions appropriate.b. Pay attention to stereochemistry unless directed otherwise and identify any racemicmixture using the symbol (±)c. Assign each reaction as addition, elimination, substitution or rearrangement, unlessdirected otherwise.arrow_forwardExplain and provide the mechanism of the reaction. Name thefinal product with the assignment of configuration.arrow_forward
- Classify each of the following cycloadditions and explain why orbital symmetry conservation rules allow or forbid each of the following reactions to occur as a concerted process.arrow_forwardDraw the frontier orbitals for the thermal and photochemical cycloreversion of this reactionarrow_forwardwith the help of symmetry properties of molecular orbitals of cyclohexadiene ,show why its con-rotatory conversion to hexatriene is thermally forbidden processarrow_forward
- 3c)Referring to the intermediates you drew in problem below explain in detail why no meta product is obtained in the Friedel-Crafts alkylation of chlorobenzene. Draw all pertinent resonance structures to support your argument.arrow_forwardExamine the following pericyclic reactions. For each reaction, tell whether it is an electrocyclic reaction, a cycloaddition reaction, or a sigmatropic rearrangement.arrow_forwardIdentify the most stable carbocation in each of the following cases and briefly justify your choicearrow_forward
- Give the structure of a, b, c, d and e in clear handwritten?arrow_forwardGive a clear handwritten answer with explanation..complete the following reactions with their stereochemistry?arrow_forwardThe spirocyclic pentadiene derivative F shown below is converted stereospecificallyinto compound G on heating. The transformation involves two consecutive pericyclicreactions of the same type, and proceeds via compound H which is not isolated. Identify the type of pericyclic reaction occurring, and determine the structure ofcompound H.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
