
Concept explainers
(a)
Interpretation: The products formed by the treatment of
Concept introduction: The
Answer to Problem 28.20P
The product formed by the treatment of
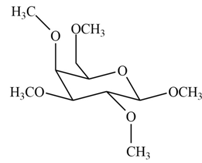
Explanation of Solution
The
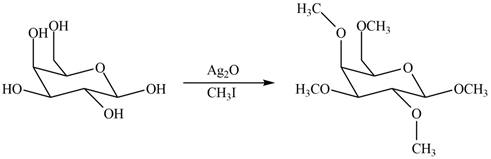
Figure 1
The products formed by the treatment of
(b)
Interpretation: The products formed by the treatment of
Concept introduction: The
Answer to Problem 28.20P
The product formed by the treatment of
Explanation of Solution
The
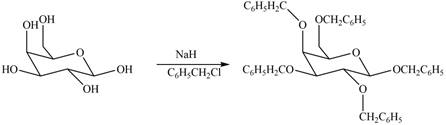
Figure 2
The products formed by the treatment of
(c)
Interpretation: The products formed by the treatment of
Concept introduction: The characteristic bond of glycoside is
Answer to Problem 28.20P
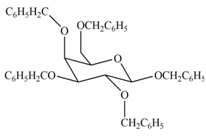
The products formed by the treatment of
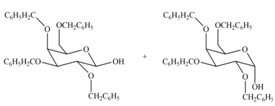
Explanation of Solution
The characteristic bond of glycoside is
The products formed by the treatment of
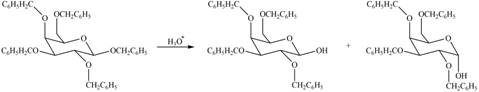
Figure 3
The products formed by the treatment of
(d)
Interpretation: The products formed by the treatment of
Concept introduction: The
Answer to Problem 28.20P
The products formed by the treatment of
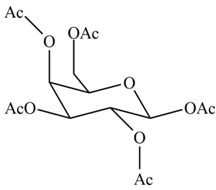
Explanation of Solution
The
The products formed by the treatment of
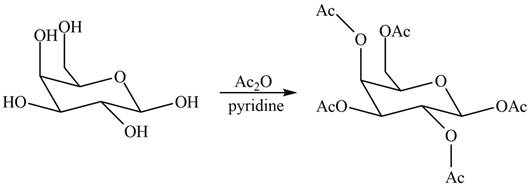
Figure 4
The products formed by the treatment of
(e)
Interpretation: The products formed by the treatment of D- galactose with
Concept introduction: The
Answer to Problem 28.20P
The product formed by the treatment of
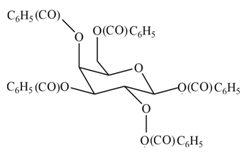
Explanation of Solution
The
The products formed by the treatment of
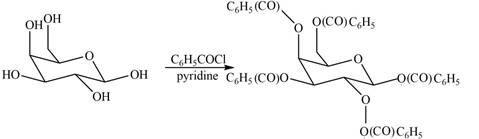
Figure 5
The products formed by the treatment of
(f)
Interpretation: The products formed by the treatment of
Concept introduction: The
Answer to Problem 28.20P
The products formed by the treatment of
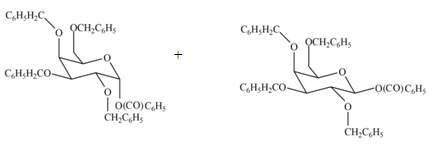
Explanation of Solution
The
The products formed by the treatment of
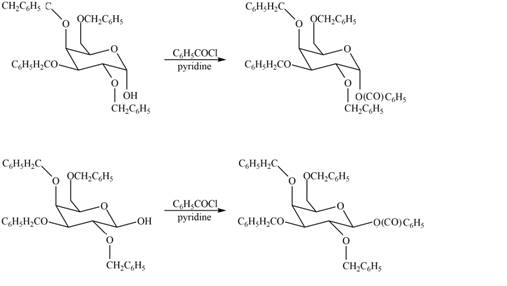
Figure 6
The products formed by the treatment of
Want to see more full solutions like this?
Chapter 28 Solutions
Organic Chemistry
- What would be the product if D-mannose is reacted witha.. 1.) NH2OH then 2.) (CH3CO)2O, NaOCOCH3 then 3.)NaOCH3, then 4.)HNO3, H2O [note this is a consectutive reaction] b. oxidation using HNO3arrow_forwardDraw the products formed when ß-D-galactose is treated with each reagent.a. Ag2O + CH3Ib. NaH + C6H5CH2Clc. The product in (b), then H3O+d. Ac2O + pyridinee. C6H5COCl + pyridinef. The product in (c), then C6H5COCl + pyridinearrow_forwardDraw the products formed when β-D-galactose is treated with below reagent. Ag2O + CH3Iarrow_forward
- 1. Show the steps in drawing the alpha pyranose of D-sorbose. 2. Show the steps in drawing the boat conformation of D-sorbose. 3. Show the steps in drawing the chair conformation of D-sorbose.arrow_forwardPredict the products obtained when d-galactose reacts with each reagent.(a) Br2 and H2O (b) NaOH, H2O (c) CH3OH, H +arrow_forwardWhat would be the product if D-allose is reacted witha.. 1.) NH2OH then 2.) (CH3CO)2O, NaOCOCH3 then 3.)NaOCH3, then 4.)HNO3, H2O [note this is a consectutive reaction] b. oxidation using HNO3arrow_forward
- Predict the products obtained when d-galactose reacts with each reagent. (h) NaBH4(i) Br2, H2O, then H2O2 and Fe2(SO4)3arrow_forward1) Phenyl propyl ether can be obtained by reacting? 2) Raffinose is A trisaccharide which is made up of two sugar molecules of aldoses and one sugar molecule of ketoses? True or false!arrow_forwardPredict the products obtained when d-galactose reacts with each reagent.(a) Br2 and H2Oarrow_forward
- a) Draw structural formulas for the two monosaccharides that result when structure III istreated as shown below. (b) Draw the monosaccharide that results when product A from the previous step (Q1a) istreated with Br2/H2O. (c) Draw the monosaccharide that results when product A from the previous step (Q1a) istreated with 1.NaBH4 / 2. H2O.arrow_forward1. Why is "A" the major acetal formed when d-erythrose reacts with H2SO4 and acetone? 2. Why do carbons 2 and 3 on molecule "A" react with H2SO4 and acetone to form acetal "B", rather than carbons 3 and 4 on molecule "A" forming a different acetal?arrow_forwardDraw the products formed when (CH3)2C=CH2 is treated with following reagent. CH3CH2OH, H2SO4arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
