
Concept explainers
Convert each compound to a Fischer projection and label each stereogenic center as
a. 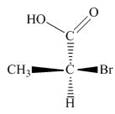 c.
c. 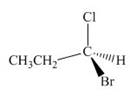 e.
e. 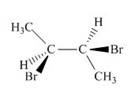
b. 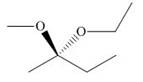 d.
d. 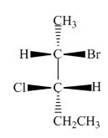 f.
f. 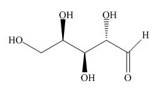
(a)
Interpretation: The given compound is to be converted into Fischer projection and the stereogenic center of a compound is to be labeled as
Concept introduction: In Fischer projection formula, the horizontal and vertical line represents the bonds that are present above and below the plane, respectively. The verticals bonds are represented as dashed wedge and horizontal bonds as dark wedge.
Answer to Problem 28.40P
The Fischer projection of given compound is,
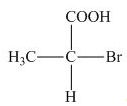
The stereogenic center of a compound is labeled as
Explanation of Solution
The structure of given compound is,
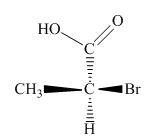
Figure 1
The horizontal and vertical line represents the bonds in front of the plane and behind the plane. Hence, the Fischer projection of given compound is shown below.
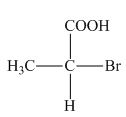
Figure 2
In the compound,
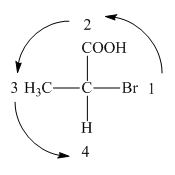
Figure 3
The circle rotates in the anticlockwise direction. Thus, the given compound is labeled as
Hence, the Fischer projection of given compound is shown in Figure 2 and the stereogenic center of a compound is labeled as
The Fischer projection of given compound is shown in Figure 2 and the stereogenic center of a compound is labeled as
(b)
Interpretation: The given compound is to be converted into Fischer projection and the stereogenic center is to be labeled as
Concept introduction: In Fischer projection formula, the horizontal and vertical line represents the bonds that are present above and below the plane, respectively. The verticals bonds are represented as dashed wedge and horizontal bonds as dark wedge.
Answer to Problem 28.40P
The Fischer projection of given compound is,
The stereogenic center of a compound is labeled as
Explanation of Solution
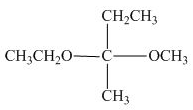
The structure of given compound is,
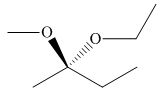
Figure 4
The given compound is redrawn. The horizontal and vertical line represents the bonds in front of the plane and behind the plane is shown below.
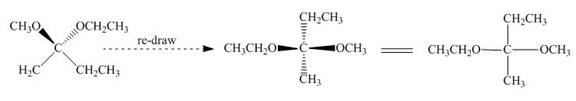
Figure 5
Hence, the Fischer projection of given compound is shown below.
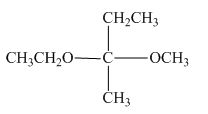
Figure 6
In the compound,
Similarly, the third and forth priorities are given to
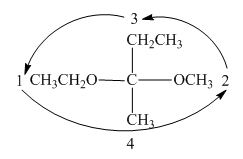
Figure 7
The circle rotates in the anticlockwise direction. Thus, the given compound is labeled as
Hence, the Fischer projection of given compound is shown in Figure 6 and the stereogenic center of a compound is labeled as
The Fischer projection of given compound is shown in Figure 6 and the stereogenic center of a compound is labeled as
(c)
Interpretation: The given compound is to be converted into Fischer projection and the stereogenic center is to be labeled as
Concept introduction: In Fischer projection formula, the horizontal and vertical line represents the bonds that are present above and below the plane, respectively. The verticals bonds are represented as dashed wedge and horizontal bonds as dark wedge.
Answer to Problem 28.40P
The Fischer projection of given compound is,
The stereogenic center of a compound is labeled as
Explanation of Solution
The structure of given compound is,
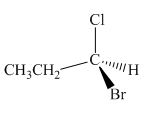
Figure 8
The given compound is redrawn. The horizontal and vertical line represents the bonds in front of the plane and behind the plane. Hence, the Fischer projection of given compound is shown below.
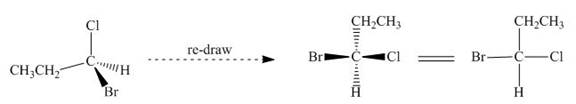
Figure 9
Hence, the Fischer projection of given compound is shown below.
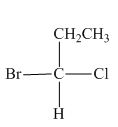
Figure 10
In the compound,
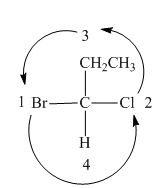
Figure 11
The circle rotates in the anticlockwise direction. Thus, the given compound is labeled as
Hence, the Fischer projection of given compound is shown in Figure 10 and the stereogenic center of a compound is labeled as
The Fischer projection of given compound is shown in Figure 10 and the stereogenic center of a compound is labeled as
(d)
Interpretation: The given compound is to be converted into Fischer projection and the stereogenic center is to be labeled as
Concept introduction: In Fischer projection formula, the horizontal and vertical line represents the bonds that are present above and below the plane, respectively. The verticals bonds are represented as dashed wedge and horizontal bonds as dark wedge.
Answer to Problem 28.40P
The Fischer projection of given compound is,
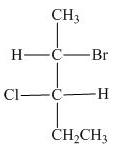
The stereogenic center of a compound are labeled as
Explanation of Solution
The structure of given compound is,
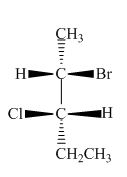
Figure 12
The horizontal and vertical line represents the bonds in front of the plane and behind the plane. Hence, the Fischer projection of given compound is shown below.
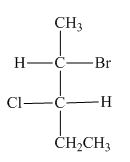
Figure 13
In the compound,
Similarly, in the
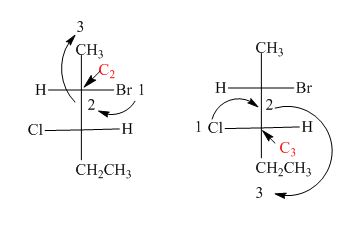
Figure 14
Hence, the Fischer projection of given compound is shown in Figure 13 and the stereogenic center of a compound are labeled as
The Fischer projection of given compound is shown in Figure 13 and the stereogenic center of a compound are labeled as
(e)
Interpretation: The given compound is to be converted into Fischer projection and the stereogenic center is to be labeled as
Concept introduction: In Fischer projection formula, the horizontal and vertical line represents the bonds that are present above and below the plane, respectively. The verticals bonds are represented as dashed wedge and horizontal bonds as dark wedge.
Answer to Problem 28.40P
The Fischer projection of given compound is,
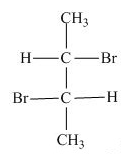
The stereogenic center of a compound as
Explanation of Solution
The structure of given compound is,
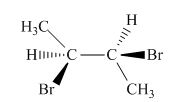
Figure 15
The given compound is redrawn. The horizontal and vertical line represents the bonds in front of the plane and behind the plane as shown below.
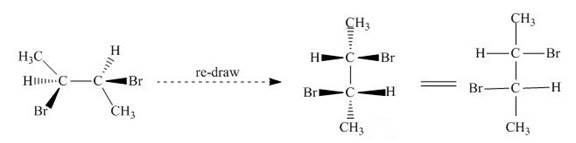
Figure 16
Hence, the Fischer projection of given compound is shown below.
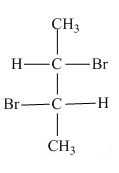
Figure 17
In the compound,
Similarly, in the
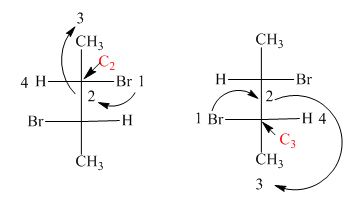
Figure 18
Hence, the Fischer projection of given compound is shown in Figure 17 and the stereogenic center of a compound are labeled as
The Fischer projection of given compound is shown in Figure 17 and the stereogenic center of a compound are labeled as
(f)
Interpretation: The given compound is to be converted into Fischer projection and the stereogenic center is to be labeled as
Concept introduction: In Fischer projection formula, the horizontal and vertical line represents the bonds that are present above and below the plane, respectively. The verticals bonds are represented as dashed wedge and horizontal bonds as dark wedge.
Answer to Problem 28.40P
The Fischer projection of given compound is,
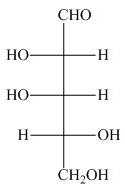
The stereogenic center of a compound are labeled as
Explanation of Solution
The structure of given compound is,
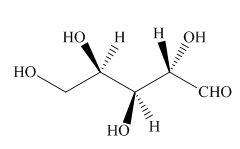
Figure 19
The Fischer projection is drawn by converting the stable staggered conformation into less stable eclipsed conformation. The bonds present below the plane in eclipsed conformation are placed on the left side of the horizontal line in Fischer projection formula as shown below.
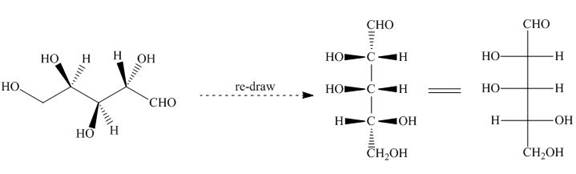
Figure 20
Hence, the Fischer projection of given compound is shown below.
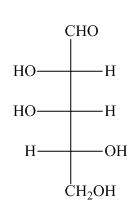
Figure21
In the compound,
Similarly, in the
Similarly, in the
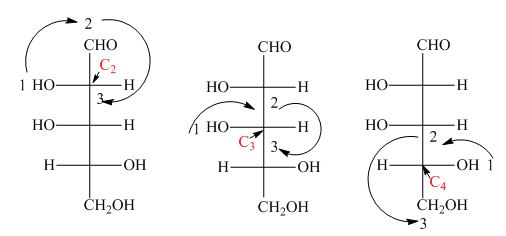
Figure 22
Hence, the Fischer projection of given compound is shown in Figure 21 and the stereogenic center of a compound are labeled as
The Fischer projection of given compound is shown in Figure 21 and the stereogenic center of a compound are labeled as
Want to see more full solutions like this?
Chapter 28 Solutions
Organic Chemistry
- Draw both pyranose anomers of each aldohexose using a threedimensionalrepresentation with a chair pyranose. Label each anomer asα or β.arrow_forwardLabel each stereogenic center as R or S (Questions a, b and c please)arrow_forwardLabel each stereogenic center as R or S (parts d, e and f please)arrow_forward
- For D-arabinose:a. Draw its enantiomer.b. Draw an epimer at C3.c. Draw a diastereomer that is not an epimer.d. Draw a constitutional isomer that still contains a carbonyl group.arrow_forwarda.Label the four stereogenic centers in sorbitol as R or S. b.How are sorbitol and A related? c. How are sorbitol and B related?arrow_forwardLabel the stereogenic center in attached compound as R or S.arrow_forward
- Label attached Haworth projection as an α or β anomer, and convert the Haworth projection to a six-membered ring with wedges and dashed wedges.arrow_forwardDraw a stepwise mechanism and all stereoisomers formed following reaction.arrow_forwardLabel each Haworth projection as an α or β anomer, and convert the Haworth projection to a six-membered ring with wedges and dashed wedges.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





