
Concept explainers
Interpretation:
The amino acid tyrosine is biologically degraded by a series of steps that include the following transformations:
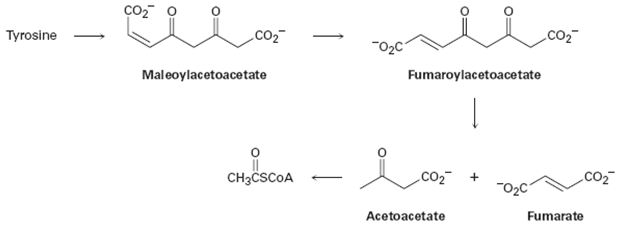
Concept introduction:
α-amino acid, in addition to their role as proton monomeric units, are energy
1) Essential amino acid
2) Non essential amino acid
While the non essential amino acid can be synchronized from metabolic processor, the essential amino acid must be provide by the diet. However, the essential dietary amino acid, instead of being extract or stored for future use, are converted to common metabolic intermediate like pyruvate, oxaloacetate, acetyl CoA, α-ketoglutarate etc. Thus amino acid are also precious of glucose, fatty acid, and
For the present, tyrosine clarifies itself to be a non essential amino acid, it is thus a metabolic processor and need not be supplied by the diet.
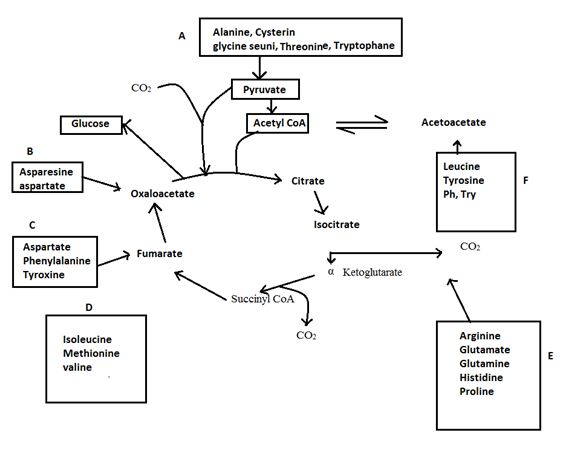
Further tyrisone is alos both a glucogenic and ketogenic amino acid. Its carbon skeleton can be degraded to either of pyruvate, α-ketoglutarate acetyl CoA, fumarate or oxaloacetate glucogenic amino acid and also to acetyl CoA or aceto acetate to be converted to ketone bodies or fatty acid. (ketogenic amino acid)
Degradation of amino acids to one of seven common metabolic intermediates
Want to see the full answer?
Check out a sample textbook solution
Chapter 29 Solutions
Organic Chemistry
- In the pentose phosphate pathway for degrading sugars, ribulose 5-phosphate is converted to ribose 5-phosphate. Propose a mechanism for the isomerization.arrow_forwardAcetolactate synthase is another TPP-requiring enzyme. It transfers the acyl group of pyruvate to another molecule of pyruvate, forming acetolactate. This is the first step in the biosynthesis of the amino acids valine and leucine. Propose a mechanism for this reaction.arrow_forwardPyruvate carboxylase is thought to activate CO2 by ATP, through formation of carboxyphosphate as an intermediate. Propose a mechanism for the formation of this intermediate.arrow_forward
- Acetolactate synthase transfers the acyl group of pyruvate to alpha-ketobutyrate. This is the first step in the biosynthesis of the amino acid isoleucine. Propose a mechanism for this reaction.arrow_forwardCarbonic anhydrase is an enzyme that catalyzes the conversion of carbon dioxide to bicarbonate ion. It is a metalloenzyme, with Zn2+ coordinated at the active site by three histidine side chains. Propose a mechanism for the reaction.arrow_forwardThe phosphoryl group on phosphoglucomutase is slowly lost by hydrolysis. Propose a mechanism that utilizes a known catalytic intermediate for restoring this essential phosphoryl group. How might this phosphoryl donor be formed?arrow_forward
- One of the steps in the metabolic degradation of guanine is hydrolysis to give xanthine. Propose a mechanism.arrow_forwardMatch the following general enzyme names and reactions catalyzed: Enzyme Reaction catalyzed a. decarboxylase b. phosphatase c. peptidase d. esterase formation of ester linkages removal of carboxyl groups from compounds hydrolysis of peptide linkages hydrolysis of phosphate ester linkagesarrow_forwardChoose among these options Among these Inhibits the enzyme which is responsible for the assembly of matured viral particles Saquinavir Didanosine Penciclovir Azidothymidine The following are essential components in the structure of sulfonamides with the exception of Amino group Benzene ring Pyridine N-Acyl amino This drug has been associated with optic neuritis and therefore discontinued after 2 months of treatment Isonizaid Rifampicin Ethambutol Pyrazinamide A non prodrug ACE Inhibitor Captopril Enalapril Quinapril Fosinopril High sodium diet High fat diet Low potassium diet Low phosphate dietarrow_forward
- In one of the steps in this pathway fructose 1,6-biphosphate (F1,6BP) is converted to gylceraldehyde-3-phosphate (G-3-p) and dihydroxyacetone phosphate (DHAP). This reaction is catalyzed by the enzyme aldolase. For this reaction at 25 celcuius and Ph7 we have: Keq= 10-4M and Delta G = +5456 cal/mol Calculate the following: The concentration of F1, 6BP, DHAP and G-3-P at equilibrium when the initial F1,6BP is (A) 1M, (b) 10-2 M, (c) 2 X 10*4 M and (d) 10-5 M. I been trying to solve this problem but I dont even know where to begin with. Atleast help me with problem A, I would be able to guide myself through.arrow_forwardDescribe the hydrolysis reaction of aspartame after the treatment of acid (HCl) and base (NaOH).Write down the mechanism and identify the compounds.arrow_forwardlodoacetamide acts as an irreversible inhibitor for a few enzymes by reacting with the amino acid at the active site having the function group -NH_2 -COOH -SH -OHarrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning



