
Concept explainers
a)
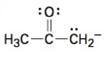
Interpretation:
To draw the maximum resonance structures possible for the species

Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species

Answer to Problem 38AP
The maximum resonance structures possible for the species  is two.
is two.
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. The anion has a carbon atom doubly bonded to an oxygen and singly bonded to an adjacent carbon atom bearing negative charge. Using the nonbonding electrons on the negatively charged carbon atom and the π bond one more structure can be drawn, as shown, without change in position or hybridization of any atom. Hence the species given has two resonance forms.
The maximum resonance structures possible for the species  are two.
are two.
b)
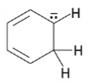
Interpretation:
To draw the maximum resonance structures possible for the species

Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species

Answer to Problem 38AP
The maximum resonance structures possible for the species  is three.
is three.
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. The anion given has a carbon atom doubly bonded to another carbon and singly bonded to an adjacent carbon atom bearing negative charge. Another double bond also is present. Using the nonbonding electrons on the negatively charged carbon atom and the π bonds two more structures can be drawn as shown, without change in position or hybridization of any atom. Hence the species given has three resonance forms.
Conclusion:
The maximum resonance structures possible for the species  are three.
are three.
The maximum resonance structures possible for the species  are three.
are three.
c)
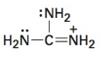
Interpretation:
To draw the maximum resonance structures possible for the species

Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species

Answer to Problem 38AP
The maximum resonance structures possible for the species  is three.
is three.
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. The cation given has a carbon doubly bonded to positively charged nitrogen and singly bonded to two more nitrogens each with a pair of nonbonding electrons. Using the nonbonding electrons on the nitrogens and the π bond in C=N, two more structures, as shown, can be drawn without change in position or hybridization of any atom. Hence the species given has three resonance forms.
The maximum resonance structures possible for the species  are three.
are three.
d)
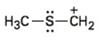
Interpretation:
To draw the maximum resonance structures possible for the species
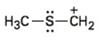
Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species
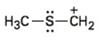
Answer to Problem 38AP
The maximum resonance structures possible for the species 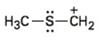 is two
is two
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. The cation given has a carbon atom singly bonded to a sulfur atom which has two lone pairs of electrons. Using the nonbonding electrons on the sulfur atom one more structure can be drawn, as shown, without change in position or hybridization of any atom. Hence the species given has two resonance forms.
The maximum resonance structures possible for the species 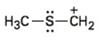 are two.
are two.
e)
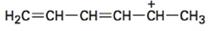
Interpretation:
To draw the maximum resonance structures possible for the species
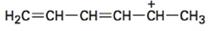
Concept introduction:
Resonance forms differ only in the placement of their π and nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. For writing different resonance forms in the structure given, first a three atom groupings with a multiple bond and a p orbital with pair of electrons is to be identified. Then the exchange of position of double bond and electrons in p orbital will give another resonance form. The shift is represented by a curved arrow.
To draw:
The maximum resonance structures possible for the species
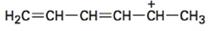
Answer to Problem 38AP
The maximum resonance structures possible for the species 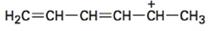 is three.
is three.
Explanation of Solution
Resonance forms differ only in the placement of their π and their nonbonding valence electrons. Neither the position nor the hybridization of any atom changes from one resonance form to another. In the cation given, the positively charged carbon is attached a carbon chain that contains two conjugated double bonds. Using the two π bonds two more structures can be drawn, as shown, without change in position or hybridization of any atom. Hence the species given has three resonance forms.
The maximum resonance structures possible for the species 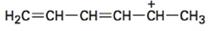 are three.
are three.
Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry
- Among the four structures in the choices, one is not a valid resonance form. Identify the wrong structure.arrow_forwardHow many total resonance structures can be drawn for the following anion (include those without separation of charge)?arrow_forwardWhich of the following resonance structures is incorrectly drawn? a) A b) B c) C d) D e) both A & Darrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
