
Concept explainers
(a)
Interpretation:
The Lewis structure for the given atom has to be drawn.
Concept Introduction:
Lewis structure: The bonding between atoms in a molecule satisfies the octet rule of valence electrons and the lone pairs also exist in the molecule. The electron is represented as dot.
(a)
Explanation of Solution
For Calcium, the number of protons and electrons is calculated as follows,
Thus, the number of electrons found is
Distributing the valence electrons around the Calcium atom as follows,

(b)
Interpretation:
The Lewis structure for the given atom has to be drawn.
Concept Introduction:
Lewis structure: The bonding between atoms in a molecule satisfies the octet rule of valence electrons and the lone pairs also exist in the molecule. The electron is represented as dot.
(b)
Explanation of Solution
For Chlorine, the number of protons and electrons is calculated as follows,
Thus, the number of electrons found is
Distributing the valence electrons around the Chlorine atom as follows,

(c)
Interpretation:
The Lewis structure for the given atom has to be drawn.
Concept Introduction:
Lewis structure: The bonding between atoms in a molecule satisfies the octet rule of valence electrons and the lone pairs also exist in the molecule. The electron is represented as dot.
(c)
Explanation of Solution
For Nitrogen, the number of protons and electrons is calculated as follows,
Thus, the number of electrons found is
Distributing the valence electrons around the Nitrogen atom as follows,
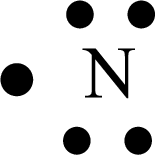
(d)
Interpretation:
The Lewis structure for the given atom has to be drawn.
Concept Introduction:
Lewis structure: The bonding between atoms in a molecule satisfies the octet rule of valence electrons and the lone pairs also exist in the molecule. The electron is represented as dot.
(d)
Explanation of Solution
For Helium, the number of protons and electrons is calculated as follows,
Thus, the number of electrons found is
Distributing the valence electrons around the Helium atom as follows,
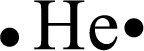
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry In Context
- What are similar and different about the Lewis structure ?arrow_forwardBased on the Lewis structures for hydrogen and helium, explain why buoyant balloons are filled with helium instead of hydrogen gas, even though hydrogen gas is cheaper and more buoyant. HHe:: a. Helium's duet makes it stable and inert (unreactive). b. Helium's duet makes it unstable and chemically reactive. c. Helium has one more electron than hydrogen, which helps add color to the balloon.arrow_forwardWhat is the geometric structure of the boron trifluoride moleCule, BF3? How many pairs of valence electrons are present on the boron atom in BF3? What are the approximate FBFbond angles in BF3?arrow_forward
- Does a Lewis structure tell which electrons came from which atoms? Explain.arrow_forwardUsing the bond energies in Table 7.2, determine the approximate enthalpy change for each of the following reactions: (a) H2(g)+Br2(g)2HBr(g) (b) CH4(g)+I2(g)CH3I(g)+HI(g) (c) C2H4(g)+3O2(g)2CO2(g)+2H2O(g)arrow_forwardWhat is the geometric structure of the SiF4molecule? How many pairs of valence electrons are present on The silicon atom of SiF4? What are the approximate FSiFbond angles in SiF4?arrow_forward
- What is the geometric sanctum of the ammonia molecule? How many pairs of electrons surround the nitrogen atom in NH3? What is the approximate HNHbond angle in ammonia?arrow_forwardrite the Lewis structure for each of the following atoms. msp;a.He(Z=2)d.Ne(Z=10)b.Br(Z=35)e.I(Z=53)c.Sr(Z=38)f.Ra(Z=88)arrow_forwardLewis Structures for Atoms Draw Lewis structures for each of the following elements. Which element is most chemically stable? a.Cb.Nec.Cad.Farrow_forward
- For three simple molecules of your own choice, apply the rules for writing Lewis structures. Write your discussion as if you are explaining the method to someone who is not familiar with Lewis structures.arrow_forwardIn bonding, what would happen between the electrons of K and Br? a.They would be transferred. b.They would be shared. c.Neither of the above. d.They would be both transferred and shared.arrow_forwardConsider the following Lewis structure where E is an unknown element: What are some possible identities for element E? Predict the molecular structure (including bond angles) for this ion. (See Exercises 25 and 26.)arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





