
Biochemistry
6th Edition
ISBN: 9781305577206
Author: Reginald H. Garrett, Charles M. Grisham
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3, Problem 13P
Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book.
Assessing the Reactivity of Carbamoyl Phosphate Consider carbamoyl phosphate, a precursor in the biosynthesis of pyrimidines:
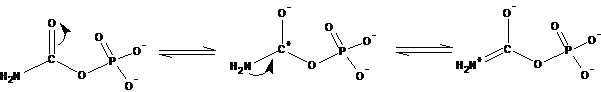
Based on the discussion of high-energy phosphates in this chapter, would you expect carbamoyl phosphate to possess a high free energy of hydrolysis? Provide a chemical rationale for your answer.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
Biochemistry
Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at (he end of this...Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Prob. 9PCh. 3 - Answers to all problems are at the end of this...
Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at the end of this...Ch. 3 - Answers to all problems are at the end of this...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Determining the Branch Points and Reducing Ends of Amylopectin A 0.2-g sample of amylopectin was analyzed to determine the fraction of the total glucose residues, that are branch points in the structure. The sample was exhaustively methylated and then digested, yielding 50-mol of 2,3-dimethylgluetose and 0.4 mol of 1,2,3,6- letramethylglucose. What fraction of the total residues are branch points? I low many reducing ends does this sample of amylopectin have?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Graphical Analysis of MWC Allosteric Enzyme Kinetics (Integrates with Chapter 1.1) Draw both Line weaver-Burk plots and Hanes-Woolf plots for an MWC allosteric enzyme system, showing separate curves for the kinetic response in (a) the absence of any effectors, (b) the presence of allosteric activator Λ, and (c) the presence of allosteric inhibitor I.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Quantitative Relationships Between Rate Constants to Calculate Km, Kinetic Efficiency (kcat/Km) and Vmax - I Measurement of the rate constants for a simple enzymatic reaction obeying Michaelis-Menten kinetics gave the following results: k1=2108M1sec1k1=1103sec1k2=5103sec1a. What is Ks, the dissociation constant for the enzyme-substrate complex? b. What is Km, the Michaelis constant for this enzyme? c. What is kcat (the turnover number) for this enzyme? d. What is the catalytic efficiency (kcat/Km) for this enzyme? e. Does this enzyme approach kinetic perfection? (That is, does kcat/Km approach the diffusion-controlled rate of enzyme association with substrate?) f. If a kinetic measurement was made using 2 nanomoles of enzyme per mL and saturating amounts of substrate, what would Vmax equal? g. Again, using 2 nanomoles of enzyme per mL of reaction mixture, what concentration of substrate would give v = 0.75 Vmax? h. If a kinetic measurement was made using 4 nanomoles of enzyme per mL and saturating amounts of substrate, what would Vmax equal? What would Km equal under these conditions?arrow_forward
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Quantitative Relationships Between Rale Constants to Calculate Km, Kinetic Efficiency (kcat/Km) and Vmax - II Triose phosphate isomerase catalyzes the conversion of glyceraldehyde-3-phosphate to dihydroxy-acetone phosphate. Glyceraldehyde3PdihydroxyacetonePThe Km of this enzyme tor its substrate glyceraldehyde-3-phosphate is 1.8 10-5 M. When [glyceraldehydes-3-phosphate] = 30 M, the rate of the reaction, v, was 82.5 mol mL-1 sec-1. a. What is Vmax for this enzyme? b. Assuming 3 nanomoles per mL of enzyme was used in this experiment ([Etotal]) = 3 nanomol/mL), what is kcat for this enzyme? c. What is the catalytic efficiency (kcat/Km) for triose phosphate isomerase? d. Does the value of kcat/Km reveal whether triose phosphate isomerase approaches catalytic perfection? e. What determines the ultimate speed limit of an enzyme-catalyzed reaction? That is, what is it that imposes the physical limit on kinetic perfection?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Assessing the Formation and Composition of Limit Dextrins Prolonged exposure of amylopectin to starch phosphorylase yields a substance called a limit dextrin. Describe the chemical composition of limit dextrins. and draw a mechanism for the enzyme-catalyzed rcactioa that can begin the breakdown of a limit dextrin.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. How Varying the Amount of Enzyme or the Addition of Inhibitors Affects v Versus [S] Plots Using Figure 13.7 as a model, draw curves that would be obtained in v versus [S] plots when a. twice as much enzyme is used. b. half as much enzyme is used. c. a competitive inhibitor is added. d. a pure noncompetitive inhibitor is added. e. an uncompetitive inhibitor is added. For each example, indicate how Vmax and Km change.arrow_forward
- Answers to all problems are at the end of this book.. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Calculating the Composition of Anomeric Sugar Mixtures -D-Glucose has a specific notation, []220, of + 112.20. whereas -D-glucose has a specific notation of +18.70. What is the composition of a mixture of - and -D-glucose, which has a specific notation of 83 .U0?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Graphing the Results from Kinetics Experiments with Enzyme Inhibitors The following kinetic data were obtained for an enzyme in the absence of any inhibitor (1), and in the presence of two different inhibitors (2) and (3) at 5 mM concentration. Assume [ET] is the same in each experiment. Graph these data as Lineweaver-Burk plots and use your graph to find answers to a. and b. a. Determine Vmax and Km for the enzyme. b. Determine the type of inhibition and the K1 for each inhibitor.arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Quantitative Relationships Between Rate Constants to Calculate Km, Kinetic Efficiency (kcat/Km) and Vmax - VI The enzyme catalase catalyzes the decomposition of hydrogen peroxide: 2H2O22H2O+O2The turnover number (kcat) for catalase is 40,000,000 sec-1. The Km of catalase for its substrate H2O2 is 0.11 M. a. In an experiment using 3 nanomole/L of catalase, what is Vmax? b. What is v when [H2O2] = 0.75 M? c. What is the catalytic efficiency of catalase? d. Does catalase approach catalytic perfection?arrow_forward
- Answers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. The Effect of Carbohydrates on Proteolysis of Glycophorin (Integrates with Chapters 5n 6, and 9.) Consider the sequence of glycophorin (see Figure 9.1U), and imagine subjecting glycophorin, and also a sample of glycophorin treated to remove all sugars, to treatment with trypsin and chymotrypsin. Would the presence of sugars in the native glycophorin make ally difference to the results?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Quantitative Relationships Between Rate Constants to Calculate Km, Kinetic Efficiency (kcat/Km) and Vmax - III The citric acid cycle enzyme fumarase catalyzes the conversion of fumarate to form malate. Fumarate+H2OmalateThe turnover number, kcat, for fumarase is 800/sec. The Km of fumarase for its substrate fumarate is 5M. a. In an experiment using 2 nanomole/mL of fumarase, what is Vmax? b. The cellular concentration of fumarate is 47.5 M. What is v when [fumarate] = 47.5 M? c. What is the catalytic efficiency of fumarase? d. Does fumarase approach catalytic perfection?arrow_forwardAnswers to all problems are at the end of this book. Detailed solutions are available in the Student Solutions Manual, Study Guide, and Problems Book. Graphical Analysis of Negative Gooperativity in KNF Allosteric Enzyme Kinetics The KNF model for allosteric transitions includes the possibility of negative cooperativity Draw Lineweaver-Burk and Hanes-Woolf plots for the case of negative cooperatively m substrate binding. (As a point of reference, include a line showing the classic Michaelis-Menten response of v to [S].)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning
Metabolism and ATP; Author: Professor Dave Explains;https://www.youtube.com/watch?v=-6VyMFQ7rRo;License: Standard YouTube License, CC-BY
Metabolic Diversity (photo-/chemo-, auto-/hetero-, litho-/organo-trophy) | GEO GIRL; Author: GEO GIRL;https://www.youtube.com/watch?v=vyLAm1CYIsI;License: Standard YouTube License, CC-BY