
Concept explainers
Three processes that have been used for the industrial manufacture of acrylonitrile (CH2CHCN), an important chemical used in the manufacture of plastics, synthetic rubber, and fibers, are shown below. Use bond energy values (Table 3-3) to estimate ∆E for each of the reactions.
a. 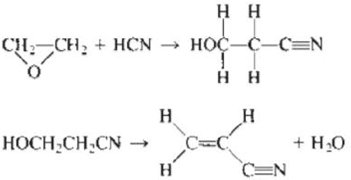
b. 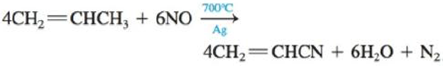
The nitrogen-oxygen bond energy in nitric oxide (NO) is 630. kJ/mol.
c. 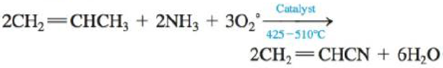
(a)
Interpretation: The change in energy for the given chemical reactions has to be calculated.
Concept introduction: In a chemical reaction, energy is gained, endothermic reactions, or released, exothermic reactions. The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
To determine: The change in energy for the stated reactions.
Answer to Problem 152CP
The required energy change is
Explanation of Solution
Given
The chemical reaction involved is,
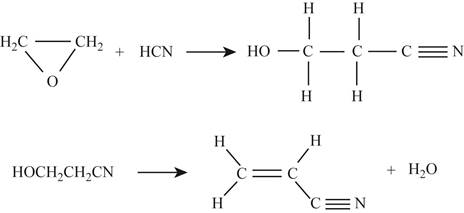
Figure 1
Formula
In the first reaction,
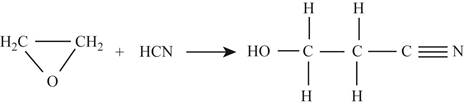
Figure 2
For first reactant,
Hence, the total energy required
For
Hence, the total energy required
Now, the total energy required for the reactants combined
Product bonds,
Hence,
The total energy released when the product is formed
So the change in energy for the first reaction is,
In the second reaction,
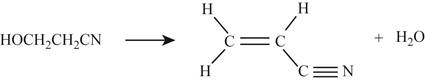
Figure 3
For the reactant,
Hence,
The total energy released when the product is formed
For product,
Hence,
The total energy released when the product is formed
For water,
So, the total energy of products
So the change in energy for the second reaction is,
The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
(b)
Interpretation: The change in energy for the given chemical reactions has to be calculated.
Concept introduction: In a chemical reaction, energy is gained, endothermic reactions, or released, exothermic reactions. The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
To determine: The change in energy for the stated reactions.
Answer to Problem 152CP
The required energy change is
Explanation of Solution
Given
The given reaction is,

Figure 4
For the reactant side,
For
The energy required
For
Total reactant energy
For products,
For
The total energy is
For
Since
For
The total energy for products is
So the change in energy for the second reaction is,
The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
(c)
Interpretation: The change in energy for the given chemical reactions has to be calculated.
Concept introduction: In a chemical reaction, energy is gained, endothermic reactions, or released, exothermic reactions. The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
To determine: The change in energy for the stated reactions.
Answer to Problem 152CP
The required energy change is
Explanation of Solution
Given
For the given reaction,

Figure 5
Energy for reactants,
For
Total energy
(since
For
Total energy
(since
For
The total energy of reactants
Energy for products,
For
The total energy
(since
For
Since
The total energy for products
So the change in energy for the second reaction is,
The change in energy can be stated as the difference between the energy required to break the bonds in case of reactants and the energy released on the formation of the products.
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry: An Atoms First Approach
- Lewis structures can be used to understand why some molecules react in certain ways. Write the Lewis structures for the reactants and products in the reactions described below. a. Nitrogen dioxide dimerizes to produce dinitrogen tetroxide. b. Boron trihydride accepts a pair of electrons from ammonia, forming BH3NH3. Give a possible explanation for why these two reactions occur.arrow_forwardHydrogenation reactions, which involve the addition of H2 to a molecule, are widely used in industry to transform one compound into another. For example, 1-butene (C4H8) is converted to butane (C4H10) by addition of H2. Use the bond dissociation enthalpies in Table 8.8 to estimate the enthalpy change for this hydrogenation reaction.arrow_forwardAcrolein is used to make plastics. Suppose this compound can be prepared by inserting a carbon monoxide molecule into the CH bond of ethylene. (a) Which is the stronger carbon-carbon bond in acrolein? (b) Which is the longer carbon-carbon bond in acrolein? (c) Is ethylene or acrolein polar? (d) Use bond dissociation enthalpies to predict whether the reaction of CO with C2H4 to give acrolein is endothermic or exothermic.arrow_forward
- Dinitrogen monoxide, N2O, can decompose to nitrogen and oxygen gas: 2 N2O(g) 2 N2(g) + O2(g) Use bond dissociation enthalpies to estimate the enthalpy change for this reaction.arrow_forwarda Carbonyl fluoride, COF2, is an extremely poisonous gas used in organofluorine synthesis. Give the valence bond description of the carbonyl fluoride molecule. (Both fluorine atoms are attached to the carbon atom.) b Nitrogen, N2, makes up about 80% of the earths atmosphere. Give the valence bond description of this molecule.arrow_forwardEthanol can be made by the reaction of ethylene and water: H2C=CH2(g) + H2O(g) CH3CH2OH(g) Use bond dissociation enthalpies to estimate the enthalpy change in this reaction. Compare the value obtained to the value calculated from enthalpies of formation.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





