
Using Figure 3.1, determine
a. the number of grams of
b. the number of grams of water required to dissolve
c. the number of grams of water required to dissolve
d. the number of grams of water required to dissolve a mixture containing
(a)
Interpretation:
The number of grams of
Concept introduction:
Solution is a homogenous mixture of two or more components. A sample taken from any part of the solution will have the same composition as the rest of the solution. Many chemical reactions occur in water solutions.
Answer to Problem 1ASA
The number of grams of
Explanation of Solution
The given illustration of the graph is shown below.
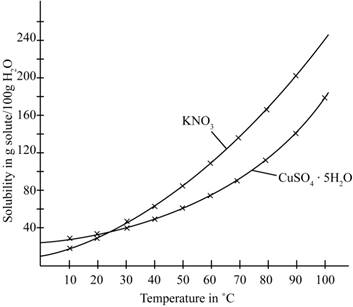
Figure 1
According to the above graph, the number of grams of
The number of grams of
(b)
Interpretation:
The number of grams of water required to dissolve
Concept introduction:
Solution is a homogenous mixture of two or more components. A sample taken from any part of the solution will have the same composition as the rest of the solution. Many chemical reactions occur in water solutions.
Answer to Problem 1ASA
The number of grams of water required to dissolve
Explanation of Solution
The given illustration of the graph is shown below.
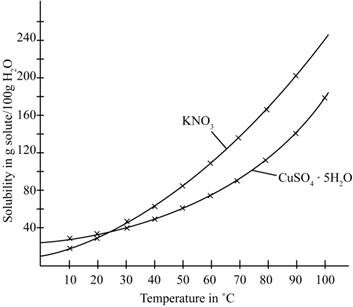
Figure 1
According to the above graph, the number of grams of
Thus, water required to dissolve
The number of grams of water required to dissolve
(c)
Interpretation:
The number of grams of water required to dissolve
Concept introduction:
Solution is a homogenous mixture of two or more components. A sample taken from any part of the solution will have the same composition as the rest of the solution. Many chemical reactions occur in water solutions.
Answer to Problem 1ASA
The number of grams of water required to dissolve
Explanation of Solution
The given illustration of the graph is shown below.
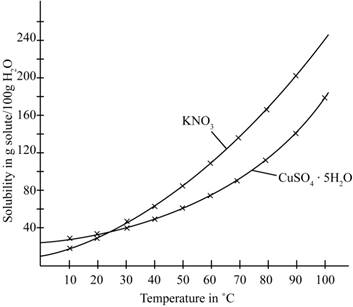
Figure 1
According to the above graph, the number of grams of
Thus, the water required to dissolve
The number of grams of water required to dissolve
(d)
Interpretation:
The number of grams of water required to dissolve a mixture containing
Concept introduction:
Solution is a homogenous mixture of two or more components. A sample taken from any part of the solution will have the same composition as the rest of the solution. Many chemical reactions occur in water solutions.
Answer to Problem 1ASA
The number of grams of water required to dissolve a mixture containing
Explanation of Solution
The given illustration of the graph is shown below.
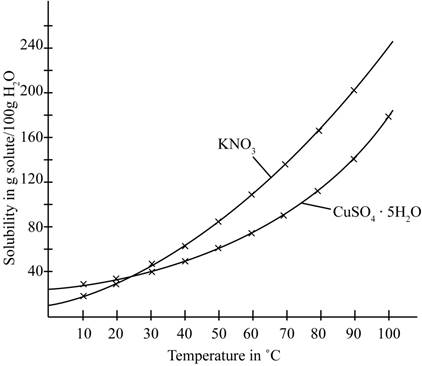
Figure 1
The amount of water required to dissolve
The amount of water required to dissolve
Therefore, the total amount of water required to dissolve a mixture containing
The number of grams of water required to dissolve a mixture containing
Want to see more full solutions like this?
Chapter 3 Solutions
Chemical Principles in the Laboratory
- You need 300. mL of 0.500-M K2Cr2O7. Which method is best to prepare this solution? Explain your choice. (a) Dilute 250. mL of 0.600-M K2Cr2O7 to 300. mL. (b) Add 50.0 mL water to 250. mL of 0.250-M K2Cr2O7. (c) Dilute 125 mL of 1.00-M K2Cr2O7 to 300. mL. (d) Add 30.0 mL of 1.50-M K2Cr2O7 to 270. mL of water.arrow_forwardCalculate the molarity of 15.4 g of sucrose (C12H22O11) in 74.0 mL of solution, [Molar mass of C12H22O11 = 342.3 g/mol] Note: Answers must be numeric, not alphanumeric—42, not forty-two.arrow_forwardWhat volume of a 18% (v/v) hydrochloric acid solution is diluted to produce 350 mL of a 5% (v/v) hydrochloric acid solution ?arrow_forward
- 1. A 50.00 mL stock CaCl2 solution was diluted with water in a 250.0-mL volumetric flask. A 25.00 mL aliquot of this was further diluted to a final volume of 100.0 mL. Finally, 20.00-mL aliquot of the resulting solution was analyzed and found to contain 0.1500 M CaCh2 Solve for the concentration of the stock solution in M. 2. A 50.00 mL stock CaCl2 solution was diluted with water in a 250.0-mL volumetric flask. A 25.00 mL aliquot of this was further diluted to a final volume of 100.0 mL. Finally, 20.00-mL aliquot of the resulting solution was analyzed and found to contain 0.1200 g CaCl2 Solve for %w/v CaCl2 in the stock solution.arrow_forwardA chemist attempts to prepare some very pure crystals of borax by dissolving 100g of Na 2 B 4 O 7 in200 grams of boiling water. He then cools the solution slowly until some borax crystallizes out.Calculate the grams of borax recovered in the crystals per 100 grams of initial solution (Na 2 B 4 O 7plus water), if the residual solution at 55 0 C after the crystals are removed contains 12.4%Na 2 B 4 O 7 . What is the percent yield or percent recovery of Na 2 B 4 O 7 . (MW: Na =23, O =16, B=10.8,H =1) round off up to 4 decimal placesarrow_forwardGeneral Chemistry Please answer all and show complete solutions. Thank you 1. What is the % volume of a 100.0 mL solution that contains 3.50 mL acetic acid? 2. Rubbing alcohol, C3H7OH, is sold as a 70.0 % v/v solution for external use only. What volume of pure C3H7OH is present in a 500.0 mL bottle? 3.What volume of a 0.25 mol/L salt solution in the kitchen contains 5.8 g of sodium chloride? 4. How many grams of sugar monosaccharide, C6H12O6, should be present in 450 g water to make a 8.75 m sugar solution? 5. What is the mole fraction of solute in a solution that has 3.50 g sucrose (MM = 342) and 100 g water (MM = 18)? 6. How many grams of sugar must be added to 1000 g water to make it boil at 102.0 degrees celsius? 7. How many grams of sugar must be added to 1000 g water to make it freeze at -2.000 degrees celsius? 8. What is the molar mass of a 500.0 g solute which then dissolved in 500.0 g water results in a solution that boils at 105.5 degrees celsius?arrow_forward
- Consider a 1.000-L stock solution of 9.69 M Na3PO4. A 2.000-mL aliquot is taken from this stock solution and diluted to a final volume of 1.000 L. A 5.000-mL aliquot is then taken from this new solution and further diluted to make a new solution with a final volume of 750.0 mL. Calculate Na+ in the final solution.arrow_forwardif i have a hypothetical ionic molecule... AB3 that dissolves in H20. and A3+ is 30 ppm...how do I determine the ppm of the other half of the molecule? 3(B-) ppm as I understand it is 1 gram of solute /1000 mL of solvent. But I don't understand what happens when that solute breaks into its anion and cation when solvating. I think it is either 10 ppm since there are 3B for every A , or it is 70 ppm so that A + B = 100%arrow_forwardUsing this graph, find the solubility of the solution (KNO3) at 35 C and 60 Carrow_forward
- What is another factor that can influence fractional distillation’s success other than the number of theoretical plates?arrow_forwardMikaela is a STEM student who is currently having her General Chemistry 2 subject.Today, she is asked by her teacher to prepare a CuSO4 solution. Help her do her task byfollowing the procedure and answering the question below.1. Obtain a sample of CuSO4 from the front bench.2. Using your scale, weigh out 18g of CuSO4.3. Dissolve the 18g of CuSO4 in 100 mL of distilled water.4. Stir until you have reached a homogeneous solution with all the solute dissolvedin the solvent.Calculate the concentration of your solution (M in mol/ L of CuSO4). Show your workin the space provided below.arrow_forward2 Compound A has a room-temperature solubility of 30.2 g/L in a certain solvent, while compound B has a solubility of 9.5 g/L in the same solvent at room temperature. An extract from a plant contains 110 g of A and 8 g of B. How much A can in principle be crystallized in pure form from this mixture if 3 L of solvent is used? How much solvent is required to dissolve the amount of B contained in this mixture? Show your steps.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
