
Concept explainers
Write balanced chemical equations for the following reactions.
(a) The reaction of aluminum and iron(III) oxide to form iron and aluminum oxide (known as the thermite reaction).
(b) The reaction of carbon and water at high temperature to form a mixture of gaseous CO and H2 (known as water gas and once used as a fuel).
(c) The reaction of liquid silicon tetrachloride and magnesium forming silicon and magnesium chloride. This is one step in the preparation of ultrapure silicon used in the semiconductor industry
(a)
Interpretation:
The balanced chemical equation for given reaction should be written.
Concept introduction:
The law of conservation of mass states that no atoms can be created or destroyed in a chemical reaction, therefore, the number of atoms present in the reactants is equal to the number of atoms present in the products.
Answer to Problem 1PS
Aluminium and iron(III) oxide reacts together and produces liquid iron and aluminum oxide which is highly exothermic reaction. The balanced equation is given below.
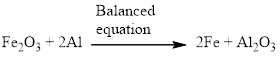
Explanation of Solution
Aluminium and iron(III) oxide reacts together and produces liquid iron and aluminum oxide which is highly exothermic reaction. The unbalanced equation is given below using correct molecular formula, the given reaction aluminium reduces the oxide of iron.

Balance the aluminum atom in the given equation, when balancing the equation, we should not alter the subscripts and we can change coefficients.
There is one aluminium in the left side and two aluminium in the right side. Therefore according to the aluminium atom the balanced equation is given below.

Next, balance the iron atom in the equation, there are two iron atom in the left side and one iron atom in right side. For the balancing the iron atom, two molecules of iron is added to the right side. Therefore according to the iron atom the balanced equation is given below.

Now the equation is balanced, therefore the balanced equation is given below,
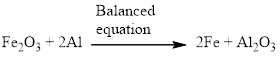
(b)
Interpretation:
The balanced chemical equation for given reaction should be written.
Concept introduction:
The law of conservation of mass states that no atoms can be created or destroyed in a chemical reaction, therefore, the number of atoms present in the reactants is equal to the number of atoms present in the products.
Answer to Problem 1PS
The balanced equation is given below (b)

Explanation of Solution
Carbon monoxide reacts with water vapor which forms the carbon dioxide and hydrogen. The mixture of carbon monoxide and hydrogen is called as water gas. This reaction is called as water-gas shift reaction (WGSR).
The unbalanced equation is given below using correct molecular formula, the given reaction Carbon monoxide reacts with water vapor which forms the carbon dioxide and hydrogen, and therefore the balanced equation is given below,

(c)
Interpretation:
The balanced chemical equation for given reaction should be written.
Concept introduction:
The law of conservation of mass states that no atoms can be created or destroyed in a chemical reaction, therefore, the number of atoms present in the reactants is equal to the number of atoms present in the products.
Answer to Problem 1PS
Silicon tetrachloride and magnesium reacts together and produces silicon and magnesium chloride. The balanced equation is given below (c)
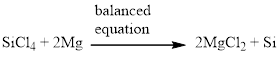
Explanation of Solution
Silicon tetrachloride and magnesium reacts together and produces silicon and magnesium chloride. The unbalanced equation is given below using correct molecular formula,
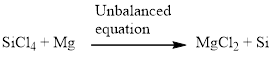
Balance the magnesium atom in the given equation, when balancing the equation, we should not alter the subscripts and we can change coefficients.
There is one magnesium atom in the left side and one magnesium in the right side. Therefore according to the magnesium there is no change in the equation. The balanced equation is given below.
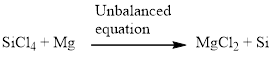
Next, balance the silicon atom in the equation, there is one silicon atom in the left side and one silicon atom in right side. Therefore according to the silicon atom the balanced equation is given below.
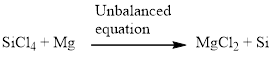
Next, balance the chlorine atom in the equation, there is four chlorine atom in the left side and two chlorine atom in the right side. Therefore 2 molecules of magnesium chloride is added to the right side of the reaction. When changing the magnesium chloride the number of magnesium is also changed therefore two molecules of magnesium is added to the left side of the reaction.
Now the equation is balanced, therefore the balanced equation is given below,
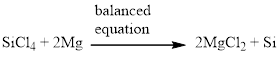
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry & Chemical Reactivity
- Write a balanced equation for (a) the combustion (reaction with oxygen gas) of glucose, C6H12O6, to give carbon dioxide and water. (b) the reaction between xenon tetrafluoride gas and water to give xenon, oxygen, and hydrogen fluoride gases. (c) the reaction between aluminum and iron(III) oxide to give aluminum oxide and iron. (d) the formation of ammonia gas from its elements. (e) the reaction between sodium chloride, sulfur dioxide gas, steam, and oxygen to give sodium sulfate and hydrogen chloride gas.arrow_forwardPhosphoric acid, H3PO4, can be prepared by the reaction of phosphorus(V) oxide, P4O10, with water. 14P4O10(s)+32H2O(l)H3PO4(aq);H=96.2kJ What is H for the reaction involving 1 mol of P4O10? P4O10(s)+6H2O(l)4H3PO4(aq)arrow_forwardYou take 1.00 g of an aspirin tablet (a compound consisting solely of carbon, hydrogen, and oxygen), burn it in air, and collect 2.20 g CO2 and 0.400 g H2O. You know that the molar mass of aspirin is between 170 and 190 g/mol. Reacting 1 mole of salicylic acid with I mole of acetic anhydride (C4H6O3) gives you 1 mole of aspirin and 1 mole of acetic acid (C2H4O2). Use this information to determine the molecular formula of salicylic acid.arrow_forward
- Bacterial digestion is an economical method of sewage treatment. The reaction is an intermediate step in the conversion of the nitrogen in organic compounds into nitrate ions. What mass of bacterial tissue is produced in a treatment plant for every 1.0 104 kg of wastewater containing 3.0% NH4+ ions by mass? Assume that 95% of the ammonium ions are consumed by the bacteria.arrow_forwardTungsten (W) metal, which is used to make incandescent bulb filaments, is produced by the reaction WO3+3H23H2O+W How many grams of H2 are needed to produce 1.00 g of W?arrow_forwardWrite balanced chemical equations for the following reactions: (a) zinc metal heated in a stream of oxygen gas (b) zinc carbonate heated until loss of mass stops (c) zinc carbonate added to a solution 0f acetic acid, CH3CO2H (d) zinc added to a solution of hydro-bromic acidarrow_forward
- Many over-the-counter antacid tablets are now formulated using calcium carbonate as the active ingredient, which enables such tablets to also be used as dietary calcium supplements. As an antacid for gastric hyperacidity, calcium carbonate reacts by combining with hydrochloric acid found in the stomach, producing a solution of calcium chloride, converting the stomach acid to water, and releasing carbon dioxide gas (which the person suffering from stomach problems may feel as a “burp”). Write the unbalanced chemical equation for this process.arrow_forwardMany cereals are made with high moisture content so that the cereal can be formed into various shapes before it is dried. A cereal product containing 58% H2O by mass is produced at the rate of 1000. kg/h. What mass of water must be evaporated per hour if the final product contains only 20.% water?arrow_forward3.14 A number of compounds are used in cement, and reactions among them occur when water is added. In one, CaO reacts with Al2O3 and water to form Ca3Al2(OH)12. Write a bal- anced chemical equation for this process.arrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





