
Concept explainers
(a)
Interpretation:
Product and balanced net ionic equation for the given aqueous solution should be written.
Concept introduction:
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water but some of the alkali metal hydroxides,
(a)
Answer to Problem 28PS
Product and balanced net ionic equation for the given aqueous solution is,
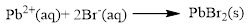
Explanation of Solution
The given compound is lead nitrate and potassium bromidewhich is soluble in water. In this reaction Pb2+ and K+ cations exchange the anions (NO3- and Br-) to give lead bromide and potassium nitrate.
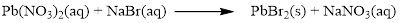
Balance the equation,
Balance the bromine atom in the given equation, when balancing the equation, we should not alter the subscripts and we can change coefficients.
There are two bromine atoms in the right side and one bromine atom in the left side. Therefore two molecule of sodium bromide is added to left side of reaction. Therefore the balanced equation is given below.
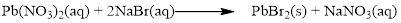
Balance the sodium atom in the given equation, there are two sodium atoms in the left side and one sodium atom in the right side. Therefore two molecule of sodium nitrate is added to right side of reaction. Therefore the balanced equation is given below.
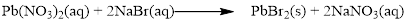
Therefore the balanced equation is given below.
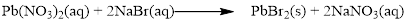
Almost all the salts of
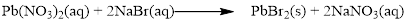
In this reaction, all the soluble ionic dissociates and forms the ions in solution.
The ionic equation is given below,

To get the net ionic equation, remove the spectator ions. Therefore the net ionic equation is given below,
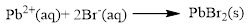
(b)
Interpretation:
Product and balanced net ionic equation for the given aqueous solution should be written.
Concept introduction:
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water but some of the alkali metal hydroxides,
(b)
Answer to Problem 28PS
Product and balanced net ionic equation for the given aqueous solution is,
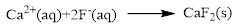
Explanation of Solution
The given compound is calcium nitrate and potassium fluoride which is soluble in water. In this reaction Ca2+ and K+ cations exchange the anions (NO3- and F-) to give calcium fluoride and potassium nitrate.
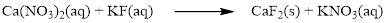
Balance the equation,
Balance the fluorine atom in the given equation, when balancing the equation, we should not alter the subscripts and we can change coefficients.
There are two fluorine atoms in the right side and one fluorine atom in the left side. Therefore two molecule of potassium fluoride is added to left side of reaction. Therefore the balanced equation is given below.
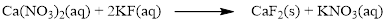
Balance the potassium atom in the given equation, there are two potassium atoms in the left side and one potassium atom in the right side. Therefore two molecule of potassium nitrate is added to right side of reaction. Therefore the balanced equation is given below.
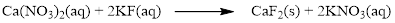
Therefore the balanced equation is given below.
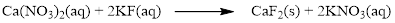
Almost all the salts of
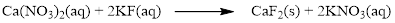
In this reaction, all the soluble ionic dissociates and forms the ions in solution.
The ionic equation is given below,
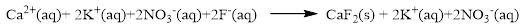
To get the net ionic equation, remove the spectator ions. Therefore the net ionic equation is given below,
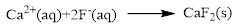
(c)
Interpretation:
Product and balanced net ionic equation for the given aqueous solution should be written.
Concept introduction:
Most of the ionic compounds are soluble in water, very few of the ionic compounds are sparingly soluble, and some of the ionic compounds are insoluble in water. When it is soluble in water ions gets separated in the solution.
Soluble compounds in water
Almost all the salts of
Almost all the salts of
Salts of F- are soluble. But some of the fluoride salt of
Salts of
Insoluble compounds in water:
Most of the salts of
Most of the metal hydroxides and oxides are insoluble in water but some of the alkali metal hydroxides,
(c)
Answer to Problem 28PS
Product and balanced net ionic equation for the given aqueous solution is,
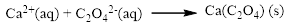
Explanation of Solution
The given compound is calcium nitrate and sodium oxalatewhich is soluble in water. In this reaction Ca2+ and Na+ cations exchange the anions (NO3- and C2O42-) to give calciumoxalate and sodium nitrate.
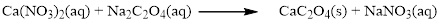
Balance the equation,
Balance the sodium atom in the given equation, when balancing the equation, we should not alter the subscripts and we can change coefficients.
There are two sodium atoms in the left side and one sodium atom in the right side. Therefore two molecule of sodium nitrate is added to right side of reaction. Therefore the balanced equation is given below.
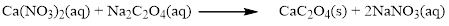
Therefore the balanced equation is given below.
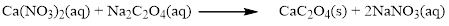
Almost all the salts of
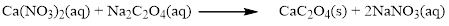
In this reaction, all the soluble ionic dissociates and forms the ions in solution.
The ionic equation is given below,
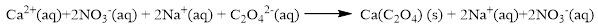
To get the net ionic equation, remove the spectator ions. Therefore the net ionic equation is given below,
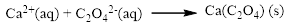
Want to see more full solutions like this?
Chapter 3 Solutions
Chemistry & Chemical Reactivity
- Vitamin C is ascorbic acid, HC6H7O6, which can be titrated with a strong base. HC6H7O6(aq) + NaOH(aq) NaC6H7O6(aq) + H2O() A student dissolved a 500.0-mg vitamin C tablet in 200.0 mL water and then titrated it with 0.1250-M NaOH. It required 21.30 mL of the base to reach the equivalence point. Calculate the mass percentage of the tablet that is impurity.arrow_forwardChlorisondamine chloride (C14H20Cl6N2) is a drug used in the treatment of hypertension. A 1.28-g sample of a medication containing the drug was treated to destroy the organic material and to release all the chlorine as chloride ion. When the filtered solution containing chloride ion was treated with an excess of silver nitrate, 0.104 g silver chloride was recovered. Calculate the mass percent of chlorisondamine chloride in the medication, assuming the drug is the only source of chloride.arrow_forwardWrite a balanced equation for the reaction of hydroiodic acid, HI, with calcium hydroxide, Ca(OH)2. Then, write the balanced complete ionic equation and the net ionic equation for this neutralization reaction.arrow_forward
- Azurite is a copper-containing mineral that often forms beautiful crystals. Its formula is Cu3(CO3)2(OH)2. Write balanced equation for the reaction of this mineral with hydrochloric acid.arrow_forwardA Describe how to prepare zinc chloride by (a) an add-base reaction, (b) a gas-forming reaction, and (c) an oxidation-reduction reaction. The available starting materials are ZnCO3, HCl, Cl2, HNO3, Zn(OH)2, NaCl, Zn(NO3)2, and Zn. Write complete, balanced equations for the reactions chosen.arrow_forwardA 1.345-g sample of a compound of barium and oxygen was dissolved in hydrochloric acid to give a solution of barium ion, which was then precipitated with an excess of potassium chromate to give 2.012 g of barium chromate, BaCrO4. What is the formula of the compound?arrow_forward
- The active ingredients of an antacid tablet contained only magnesium hydroxide and aluminum hydroxide. Complete neutralization of a sample of the active ingredients required 48.5 mL of 0.187 M hydrochloric acid. The chloride salts from this neutralization were obtained by evaporation of the filtrate from the titration; they weighed 0. 4200 g. What was the percentage by mass of magnesium hydroxide in the active ingredients of the antacid tablet?arrow_forwardWrite balanced net ionic equations for the following reactions in acid solution. (a) Liquid hydrazine reacts with an aqueous solution of sodium bromate. Nitrogen gas and bromide ions are formed. (b) Solid phosphorus (P4) reacts with an aqueous solution of nitrate to form nitrogen oxide gas and dihydrogen phosphate (H2PO4-) ions. (c) Aqueous solutions of potassium sulfite and potassium permanganate react. Sulfate and manganese(II) ions are formed.arrow_forwardMagnesium metal (a component of alloys used in aircraft and a reducing agent used in the production of uranium, titanium, and other active metals) is isolated from sea water by the following sequence of reactions: Mg2+(aq)+Ca(OH)2(aq)Mg(OH)2(s)+Ca2+(aq)Mg(OH)2(s)+2HCl(aq)MgCl2(s)+2H2O(l)MgCl2(l)electrolysisMg(s)+Cl2+Cl2(g) Sea water has a density of 1.026 g/cm3 and contains 1272 parts per million of magnesium a5 Mg2+(aq) by mass. What mass, in kilograms, of Ca(OH)2; is required to precipitate 99.9% of the magnesium in 1.00103 L of sea water?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





